Approach
It is important to identify respiratory failure promptly in order to initiate reversal and maximise the chances of survival and good outcome. In the emergency setting, this includes an Airway, Breathing, Circulation, Disability, Exposure (ABCDE) assessment, followed by pulse oximetry, blood gas analysis, blood tests, ECG, and chest x-ray.[1][22][23] Subsequent tests may include capnometry, toxicology, pulmonary function testing, computed tomography (CT) chest, CT pulmonary angiogram, ventilation/perfusion lung scan, and cardiothoracic ultrasound.[23][24][25][26] For those with possible cardiac failure or acute cardiac injury, cardiac ultrasound may show myocardial dyskinesis or valvular dysfunction as a contributor to acute respiratory failure.
Historical factors
Patients with acute hypoxaemic respiratory failure can present with shortness of breath (dyspnoea), but this often leads quickly to agitation, anxiety, and struggling to breathe more deeply and rapidly. As hypoxia advances, patients may have headache, confusion, somnolence, and seizure.
Patients with hypercapnia generally tolerate their symptoms better than those with hypoxia alone, as the respiratory failure develops more slowly. Hypercapnia where the arterial partial pressure of carbon dioxide (PaCO₂) has acutely risen to >10.7 kPa (>80 mmHg) may produce headache, confusion, disorientation, coma, and seizure.[1] Patients with severe hypercapnia often appear comfortable and resting while actually progressively hypoventilating and developing severe respiratory acidosis and hypoxaemia secondary to decreased respiratory effort. Friends/relatives may describe agitation, slurred speech, and tremor.
Examination findings
An ABCDE assessment is essential for all patients. This should be re-assessed regularly and include an evaluation of the airway patency and the patient's ability to protect the airway by checking the airway/gag reflex using a tongue depressor or laryngoscope blade.
Respiratory distress is usually observed in patients with acute hypoxaemic respiratory failure (type I respiratory failure). Signs of this include tachypnoea (respiratory rate >24 breaths per minute in adults), use of accessory breathing muscles, and cyanosis.[1] Without rapid and effective intervention for hypoxaemic respiratory failure, encephalopathy, cardiac dysfunction, multi-system organ failure, and death can occur.
Hypercapnic respiratory failure (type II respiratory failure) is often more difficult to recognise than hypoxaemic respiratory failure because tachypnoea is often less profound, if present at all. Early signs may be subtle and include agitation, slurred speech, asterixis, and decreased level of consciousness.[1] Failure to recognise and reverse acute hypercapnic respiratory failure can result in severe respiratory acidosis with subsequent myocardial depression, electrolyte imbalance, and multi-system organ failure.
Pulse oximetry
Pulse oximetry is measured during the examination and provides non-invasive measurements of capillary oxygen saturation (SpO₂) using transmitted and absorbed light sources. Low SpO₂ or temporarily decreasing SpO₂ can indicate impending respiratory failure. Pulse oximetry can be limited by a number of factors including anaemia, nail polish, low perfusion to oximeter attachment site, and inaccuracy in readings.[27]
Blood gas analysis
Arterial blood gases are required very early and should be obtained as soon as possible after an ABCDE assessment has been made.
Analysis provides sensitive measures of pulmonary function. Low PaO₂ (<8 kPa [<60 mmHg]) indicates hypoxaemia and supports the diagnosis of hypoxaemic respiratory failure. Elevated PaCO₂ (>6.7 kPa [>50 mmHg]) in the presence of hypoxia (PaO₂ <8 kPa [<60 mmHg]) indicates the presence of hypercapnic respiratory failure and can result in respiratory acidosis.[1] Acute hypercapnic respiratory failure is characterised by an elevated PaCO₂ and reduced pH. Chronic forms of hypercapnic respiratory failure are characterised by renal retention of bicarbonate to neutralise excess carbonic acid. This results in slightly decreased pH, elevated PaCO₂, and elevated serum bicarbonate.[28]
Blood tests
Blood tests can help to diagnose underlying causes. Samples can be taken when intravenous access is achieved either during or after an ABCDE assessment.
The serum bicarbonate (HCO₃) concentration provides information on the acid-base status. Acid-base balance is maintained within the body in large part by the interaction between PaCO₂ and HCO₃. HCO₃ is negatively charged and acts to bind free hydrogen ion (H+), which is formed in acidotic states. The renal system helps to regulate serum HCO₃, which is usually maintained at approximately 25 mEq/L. Acute metabolic acidosis usually results in a relatively quick decrease in HCO₃ concentration. Chronic respiratory acidosis, commonly seen with COPD, is associated with elevated HCO₃, which slowly develops over time. An elevated HCO₃ in association with developing acute respiratory failure implies underlying chronic obstructive lung disease as a comorbid condition.[29]
Full blood count (showing an abnormal white blood cell profile) can help detect underlying infection, as well as polycythaemia that often accompanies chronic respiratory failure.
D-dimer testing to detect possible pulmonary embolism may be of some value if the test is negative (within normal range). D-dimer testing is limited because of the potential for significant false positives.[30]
Cardiac troponin I and T should be considered if a cardiac aetiology for acute respiratory failure is a potential.
ECG
ECG can be done following the ABCDE assessment.[23] This will help in diagnosing the underlying cardiac causes and occasionally pulmonary embolism.
Chest x-ray
A portable chest x-ray can be done once an ABCDE assessment has been made and the patient is sufficiently stable. This will help in diagnosing the underlying cause.
Diffuse or patchy infiltrates on chest x-ray can be associated with pneumonia, pulmonary oedema, aspiration, progressive interstitial lung disease, pulmonary contusion, and alveolar haemorrhage. Minimal changes on chest x-ray are often seen in asthma, pulmonary embolism, and respiratory depression. When assessing chest x-rays of patients with chronic lung disease, previous radiographs are usually needed for comparison and to determine if acute changes are present.
Capnometry
Capnometry, which is usually done in association with endotracheal intubation, measures expired CO₂ and reflects arterial CO₂. Measurements are dependent on pulmonary perfusion status (cardiac output and alveolar blood flow patterns). The two types of capnograph that are commonly used are mainstream capnographs, which employ a CO₂ sensor located in the breathing circuit (usually between the endotracheal tube and the air stream mechanical ventilator connectors), and side-stream capnography, where the CO₂ sensor is located away from the breathing circuit (usually connected to a side tube, which is connected to the breathing circuit to sample CO₂ within the breathing circuit). Side-stream capnography can be used when patients are not intubated and is becoming more commonly used in assessment and monitoring of patients with acute illness or injury, to detect worsening respiratory condition.
Pulmonary function tests
Peak expiratory flow rates (PEFR) and/or forced expiratory volumes (FEV) of less than predicted values indicate airflow obstruction. Monitoring the trend in these pulmonary function tests provides clinical indication of improvement or deterioration in respiratory function. Bedside spirometry can provide forced vital capacity (FVC), a measure of lung volume versus time or flow versus volume. FVC can be helpful in the assessment of the severity of obstructive pulmonary disease. Reduction in both FEV and FVC suggests restrictive lung disease. Respiratory failure from obstructive disease is not expected when the FEV in 1 second (FEV1) is >1 litre and in restrictive disease when the FVC is >1 litre.
How to use a peak flow meter to obtain a peak expiratory flow measurement.
Bedside negative inspiratory force (NIF) can be used in the critical care setting to assess the strength of inspiration. Poor NIF can be predictive of impending respiratory failure in situations where neuromuscular dysfunction is suspected. NIF can also be used to predict success with planned extubation of patients.
Pulmonary function testing is often most helpful when sequential testing is compared to determine improvement versus failure with therapy.
[Figure caption and citation for the preceding image starts]: Spirometry results for normal, restrictive, and obstructive patternsCreated by the BMJ Knowledge Centre [Citation ends].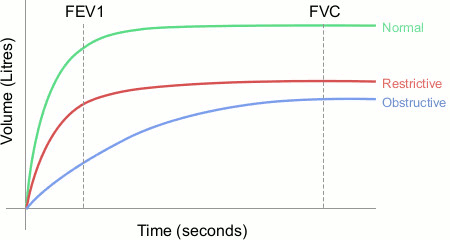
Toxicology testing
Toxicology testing of serum and urine can be helpful in confirming suspected medicine or recreational drug causes of respiratory failure (e.g., opioid overdose).
Chest CT
Chest CT may be helpful in identifying the underlying cause once the patient has stabilised: for example, pulmonary oedema, pulmonary embolism, and acute asthma exacerbation.[24] CT can reveal chronic lung disease, pulmonary consolidation and effusion, parenchymal disease, bronchiectasis, and pulmonary embolism involving large- and medium-sized pulmonary arteries.
A CT scan may not be sensitive enough to detect small pulmonary vessel embolism. In one retrospective study involving patients with suspected acute pulmonary embolism, a CT scan explained the clinical presentation in approximately 50% of patients, but there were negative findings in around half of patients.[34] The need to transport a patient to CT scanner equipment limits the use of this modality in critically ill individuals. Patients must also be tolerant of the momentary breath-holding required to obtain the CT images.
CT pulmonary angiography (CTPA)
CTPA is the imaging study of choice in patients with an abnormal D-dimer or a high probability of pulmonary embolism.[35] A CTPA uses contrast medium, which is administered intravenously at the same time as the CT scan of the chest. This allows filling defects to be visualised in the segmental and subsegmental branches of the pulmonary circulation. It has the highest diagnostic accuracy for pulmonary embolism of all the advanced non-invasive imaging methods.[35] However, a CTPA is contraindicated in patients who have an allergy to contrast media or have renal failure.[36] It should also be avoided in pregnant women.[37]
Ventilation/perfusion (V/Q) lung scanning
V/Q lung scan, preferably using single photon emission computed tomography (SPECT, which may reduce the number of inconclusive scans), is an alternative to CTPA.[38] A negative V/Q scan effectively excludes pulmonary embolism. The sensitivity and specificity of a V/Q scan is dependent upon patient physical characteristics and operator accuracy, but a correctly performed negative V/Q scan is considered to clinically rule out a large vessel pulmonary embolism due to its high negative predictive value.[26]
Cardiothoracic ultrasound
Cardiothoracic ultrasound is a complementary diagnostic tool that contributes to an early therapeutic decision based on reproducible pathophysiological data (pulmonary parenchyma consolidation, evidence of pneumothorax, and assessment of right ventricular filling and function).[25] It is a non-invasive bedside test that can be used to initiate therapy pending confirmation by more advanced imaging techniques. Thoracic ultrasound is also of use in directing needle location for thoracentesis (drainage) procedures and placement of drainage devices.[39][40]
Emerging tests
Transcutaneous CO₂ monitoring has a potential use in continuously monitoring the PaCO₂ of a patient who is being maintained with non-invasive ventilatory support.[41] At present, transcutaneous CO₂ monitoring technology is developing and current agreement between PaCO₂ and transcutaneous PCO₂ has been questioned as being suboptimal.[42]
How to obtain an arterial blood sample from the radial artery.
How to perform a femoral artery puncture to collect a sample of arterial blood.
Use of this content is subject to our disclaimer