Superior vena cava syndrome
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
acute airway obstruction (without tissue diagnosis)
secure airway + radiotherapy + corticosteroids
Airway should be secured by intubation or surgically first.
Urgent treatment with radiotherapy and corticosteroids should be used only for life-threatening situations. It should be deferred otherwise, due to interference with subsequent histopathological diagnosis.
Dose of corticosteroids should be limited and the dose decreased after 1 to 2 days of treatment or following symptomatic improvement.
Primary options
dexamethasone: 10 mg intravenous bolus initially, followed by 4 mg every 6 hours
secure airway + percutaneous endovascular stenting
Airway should be secured by intubation or surgically first.
Becoming increasingly used, because the stent can be placed before a tissue diagnosis is available. It is a useful procedure for patients with severe symptoms such as respiratory distress that require urgent intervention.[21]Urruticoechea A, Mesia R, Dominguez J, et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion: experience on 52 patients with lung cancer. Lung Cancer. 2004;43:209-214. http://www.ncbi.nlm.nih.gov/pubmed/14739042?tool=bestpractice.com [22]Schifferdecker B, Shaw JA, Piemonte TC, et al. Nonmalignant superior vena cava syndrome: pathophysiology and management. Catheter Cardiovasc Interv. 2005;65:416-423. http://www.ncbi.nlm.nih.gov/pubmed/15926179?tool=bestpractice.com Meta-analyses have demonstrated that endovascular therapy with stenting has high technical and clinical success rates.[23]Azizi AH, Shafi I, Zhao M, et al. Endovascular therapy for superior vena cava syndrome: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jul;37:100970. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8343254 http://www.ncbi.nlm.nih.gov/pubmed/34386747?tool=bestpractice.com [24]Aung EY, Khan M, Williams N, et al. Endovascular stenting in superior vena cava syndrome: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2022 Sep;45(9):1236-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9458578 http://www.ncbi.nlm.nih.gov/pubmed/35821122?tool=bestpractice.com [25]Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006 May-Jun;29(3):319-22. http://www.ncbi.nlm.nih.gov/pubmed/16502166?tool=bestpractice.com
Done percutaneously by obtaining access usually through the femoral vein. Performed under conscious sedation. Fluoroscopic guidance and iodinated contrast are used. Most operators use heparin during the procedure. [Figure caption and citation for the preceding image starts]: Post-dilatation of the superior vena cava stentImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].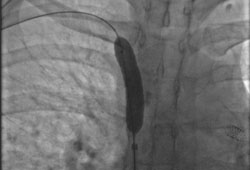 [Figure caption and citation for the preceding image starts]: Venography showing superior vena cava stenosis. Stent placement in the left pulmonary artery is seenImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Venography showing superior vena cava stenosis. Stent placement in the left pulmonary artery is seenImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Percutaneous balloon angioplasty of the stenotic lesion in superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Percutaneous balloon angioplasty of the stenotic lesion in superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].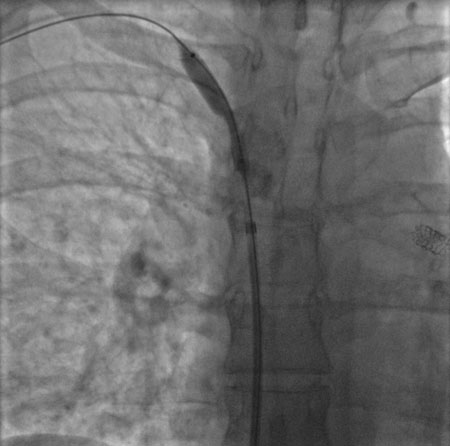 [Figure caption and citation for the preceding image starts]: Stent deployment in the superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Stent deployment in the superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
Self-expanding or balloon-expandable stents may be used (usually bare metal stents).
Complications of percutaneous stenting are in the range of 3% to 7% and include volume overload due to sudden increase in preload, stent thrombosis, pulmonary embolus, stent migration, haematoma at the insertion site, infection, bleeding, and, very rarely, perforation or death.[22]Schifferdecker B, Shaw JA, Piemonte TC, et al. Nonmalignant superior vena cava syndrome: pathophysiology and management. Catheter Cardiovasc Interv. 2005;65:416-423. http://www.ncbi.nlm.nih.gov/pubmed/15926179?tool=bestpractice.com
Patency rate is around 80% to 94%, and 20% of patients may require repeat stenting.
Bleeding risk is 1% to 14%, due to anticoagulation with aspirin, clopidogrel, and/or warfarin, which may be used following stent placement to prevent thrombosis.[21]Urruticoechea A, Mesia R, Dominguez J, et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion: experience on 52 patients with lung cancer. Lung Cancer. 2004;43:209-214. http://www.ncbi.nlm.nih.gov/pubmed/14739042?tool=bestpractice.com [31]Courtheoux P, Alkofer B, Al Refai M, et al. Stent placement in superior vena cava syndrome. Ann Thorac Surg. 2003;75:158-161. http://www.ncbi.nlm.nih.gov/pubmed/12537210?tool=bestpractice.com
Data regarding post-procedural anticoagulation are lacking, and practices vary.[2]Azizi AH, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020 Dec 28;13(24):2896-2910. https://www.sciencedirect.com/science/article/pii/S1936879820319725?via%3Dihub http://www.ncbi.nlm.nih.gov/pubmed/33357528?tool=bestpractice.com
malignant aetiology
treatment of malignancy
Most malignant tumours causing superior vena cava (SVC) syndrome are sensitive to radiotherapy.
Chemotherapy is an effective option for treatment of lung cancer, lymphomas, and germ cell tumours.[26]Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol). 2002;14:338-351. http://www.ncbi.nlm.nih.gov/pubmed/12555872?tool=bestpractice.com
Thymomas that are resistant to chemotherapy and radiation may require surgical resection and SVC reconstruction (operative mortality rate of 5% and patency rate of 80% to 90%).[27]Detterbeck FC, Parsons AM. Thymic tumors. Ann Thorac Surg. 2004;77:1860-1869. http://www.ncbi.nlm.nih.gov/pubmed/15111216?tool=bestpractice.com
Selection of therapy will depend on the type of malignancy, staging, and histopathology. See Small cell lung cancer, Non-small cell lung cancer, Non-Hodgkin's lymphoma, Thymic tumour.
percutaneous endovascular stenting
Treatment recommended for ALL patients in selected patient group
Endovascular stenting is performed to achieve more rapid improvement in symptoms and has fewer side effects compared with radiotherapy.[23]Azizi AH, Shafi I, Zhao M, et al. Endovascular therapy for superior vena cava syndrome: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jul;37:100970. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8343254 http://www.ncbi.nlm.nih.gov/pubmed/34386747?tool=bestpractice.com [24]Aung EY, Khan M, Williams N, et al. Endovascular stenting in superior vena cava syndrome: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2022 Sep;45(9):1236-54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9458578 http://www.ncbi.nlm.nih.gov/pubmed/35821122?tool=bestpractice.com [25]Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006 May-Jun;29(3):319-22. http://www.ncbi.nlm.nih.gov/pubmed/16502166?tool=bestpractice.com
Undertaken percutaneously by obtaining access (usually) through the femoral vein. Performed under conscious sedation. Fluoroscopic guidance and iodinated contrast are used and most operators use heparin during the procedure.[Figure caption and citation for the preceding image starts]: Post-dilatation of the superior vena cava stentImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Venography showing superior vena cava stenosis. Stent placement in the left pulmonary artery is seenImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Venography showing superior vena cava stenosis. Stent placement in the left pulmonary artery is seenImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Percutaneous balloon angioplasty of the stenotic lesion in superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Percutaneous balloon angioplasty of the stenotic lesion in superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Stent deployment in the superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Stent deployment in the superior vena cavaImage obtained from cardiac catheterisation laboratory at University of Missouri, Columbia; used with permission [Citation ends].
Self-expanding or balloon-expandable stents may be used (usually bare metal stents).
Complications of percutaneous stenting are in the range of 3% to 7% and include volume overload due to sudden increase in preload, stent thrombosis, pulmonary embolus, stent migration, haematoma at the insertion site, infection, bleeding, and, very rarely, perforation or death.[22]Schifferdecker B, Shaw JA, Piemonte TC, et al. Nonmalignant superior vena cava syndrome: pathophysiology and management. Catheter Cardiovasc Interv. 2005;65:416-423. http://www.ncbi.nlm.nih.gov/pubmed/15926179?tool=bestpractice.com
Patency rate is around 80% to 94%, and 20% of patients may require repeat stenting.
Bleeding risk is 1% to 14%, due to anticoagulation with aspirin, clopidogrel, and/or warfarin, which may be used following stent placement to prevent thrombosis.[21]Urruticoechea A, Mesia R, Dominguez J, et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion: experience on 52 patients with lung cancer. Lung Cancer. 2004;43:209-214. http://www.ncbi.nlm.nih.gov/pubmed/14739042?tool=bestpractice.com [31]Courtheoux P, Alkofer B, Al Refai M, et al. Stent placement in superior vena cava syndrome. Ann Thorac Surg. 2003;75:158-161. http://www.ncbi.nlm.nih.gov/pubmed/12537210?tool=bestpractice.com
Data regarding post-procedural anticoagulation are lacking, and practices vary.[2]Azizi AH, Shafi I, Shah N, et al. Superior vena cava syndrome. JACC Cardiovasc Interv. 2020 Dec 28;13(24):2896-2910. https://www.sciencedirect.com/science/article/pii/S1936879820319725?via%3Dihub http://www.ncbi.nlm.nih.gov/pubmed/33357528?tool=bestpractice.com
palliative therapy
Includes palliative radiotherapy, chemotherapy or corticosteroids (for lymphomas and thymomas), endovascular stents, or rarely bypass surgery, as it is invasive and difficult.[1]Wilson LD, Detterbeck FC, Yahalom J. Clinical practice: superior vena cava syndrome with malignant causes. N Engl J Med. 2007 May 3;356(18):1862-9. http://www.ncbi.nlm.nih.gov/pubmed/17476012?tool=bestpractice.com
In rare cases, surgical decompression can be performed.
Thrombolysis with indwelling catheters has also been described in small studies.[28]Gray BH, Olin JW, Graor RA, et al. Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest. 1991;99:54-59. http://www.ncbi.nlm.nih.gov/pubmed/1984986?tool=bestpractice.com
Supportive treatment consists of diuretics, low-salt diet, avoidance of upper-extremity lines, head elevation, and oxygen.
Type of diuretic will depend on several factors including renal function and current or past history of diuretic use.
infectious aetiology
treatment of infection
Underlying infection (e.g., aspergillosis, blastomycosis, histoplasmosis, nocardiosis) should be treated according to local sensitivities.
Choice of antimicrobial will depend on the underlying pathogen.
palliative therapy
Endovascular stents and more rarely bypass surgery may be required if superior vena cava obstruction persists after treatment of infection.
iatrogenic aetiology
catheter removal + thrombolysis and/or anticoagulation
Catheter(s) should be removed and local thrombolysis and/or short-course anticoagulation should be considered in these patients.[29]Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016 Feb;149(2):315-52. http://journal.chestnet.org/article/S0012-3692(15)00335-9/fulltext http://www.ncbi.nlm.nih.gov/pubmed/26867832?tool=bestpractice.com
Chronic warfarin therapy up to 1 year has been described in some reports.[32]Kee ST, Kinoshita L, Razavi MK, et al. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998;206:187-193. http://www.ncbi.nlm.nih.gov/pubmed/9423671?tool=bestpractice.com Dose should be titrated to target INR of 2.0 to 3.0.
Thrombolysis has higher success rates if used within first 5 days of development of superior vena cava obstruction.[28]Gray BH, Olin JW, Graor RA, et al. Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest. 1991;99:54-59. http://www.ncbi.nlm.nih.gov/pubmed/1984986?tool=bestpractice.com
percutaneous balloon dilatation/stenting with or without lead removal
Percutaneous balloon dilatation/stenting is preferred in these patients.
Lead explantation may carry a high risk of mortality (1% to 3%).[30]Smith CW, Messenger JC, Schaner SP, et al. Cardiac pacemaker electrodes: improved methods of extraction. Radiology. 1994;193:739-742. http://www.ncbi.nlm.nih.gov/pubmed/7972816?tool=bestpractice.com Bypass surgery may be an option.
Infection of the leads should always be considered as a possibility and evaluated with blood cultures and transoesophageal echocardiogram.
Long-term anticoagulation with warfarin should be considered. Dose should be titrated to target INR of 2.0 to 3.0.

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer