The aetiology of tachycardia is variable and often multifactorial.
The most common type is sinus tachycardia.
Cases of tachyarrhythmias not due to a heightened sinus rate may be due to focal islets of tissue that fire rapidly because of elevated automaticity, triggered activity, or re-entry within a tissue with heterogeneous conduction properties.
Secondary causes of tachyarrhythmia include ion channelopathies, myocardial scar, surgical scar, elevated atrial or ventricular wall tension and stretch due to elevated filling pressures, ischaemia, electrolyte abnormalities, elevated intrinsic catecholamines, myocarditis, or any combination of these causes. Prescribed, legitimate, and illicit drug use have been implicated.
Narrow QRS (duration <120 ms) with a regular ventricular rhythm
Sinus tachycardia
A rhythm that originates in the sino-atrial (sinus) node with a rate above 100 bpm. This is usually a normal response to physical, emotional, physiological, or pharmacological stress. Secondary causes of sinus tachycardia include physical deconditioning, hypoxia, pulmonary embolism, hypovolaemia, hyperthyroidism, anaemia, drugs (e.g., caffeine, alcohol, nicotine, amphetamines, cocaine), and prescribed medications (e.g., aminophylline, atropine, clozapine, catecholamines).[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
[19]Lally J, Docherty MJ, MacCabe JH. Pharmacological interventions for clozapine-induced sinus tachycardia. Cochrane Database Syst Rev. 2016;(6):CD011566.
http://www.ncbi.nlm.nih.gov/pubmed/27277334?tool=bestpractice.com
Postural orthostatic tachycardia syndrome (POTS)
A chronic, multi-system disorder that is thought to be due to an autoimmune process. POTS is characterised by:[20]Vernino S, Bourne KM, Stiles LE, et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton Neurosci. 2021 Nov;235:102828.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8455420
http://www.ncbi.nlm.nih.gov/pubmed/34144933?tool=bestpractice.com
[21]Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015 Jun;12(6):e41-63.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5267948
[22]Shen WK, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society. Circulation. 2017 Aug 1;136(5):e25-59.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000498
[23]Raj SR, Guzman JC, Harvey P, et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020 Mar;36(3):357-72.
https://onlinecjc.ca/article/S0828-282X(19)31550-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/32145864?tool=bestpractice.com
frequent symptoms of orthostatic intolerance (that improve rapidly when the patient returns to a supine position) that interfere with daily living activities, and have continued for at least 3 months, and
an increase in heart rate by ≥30 bpm (or ≥40 bpm in patients aged 12 to 19 years) within 10 minutes of standing from a supine position or head-up tilt (without orthostatic hypotension) that is not due to other causes of sinus tachycardia.
Inappropriate sinus tachycardia
A persistent increase in resting heart rate unrelated to or out of proportion to physical, emotional, pathological, or pharmacological stress (resting heart rate >100 bpm or average heart rate >90 bpm in 24-hour ECG monitoring). The precise aetiology is unknown and is likely to be multifactorial. Elevated sinus node automaticity and autonomic dysfunction have been proposed as possible causes.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
[21]Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015 Jun;12(6):e41-63.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5267948
Atrial tachycardia
Rapid atrial activation from a region of the atria other than the sinus node with rates typically between 100 and 250 bpm. Multifocal atrial tachycardia is defined as three or more sites of atrial activation (commonly irregular rhythm). Focal atrial tachycardia can occur without cardiac disease. Atrial tachycardia with AV block should raise the suspicion for digitalis toxicity. Hypokalaemia can also exacerbate this condition.[24]Steinbeck G, Hoffmann E. 'True' atrial tachycardia. Eur Heart J. 1998 May;19(Suppl E):E10-2,E48-9.
http://www.ncbi.nlm.nih.gov/pubmed/9717019?tool=bestpractice.com
Right-sided atrial tachycardias tend to originate from the crista terminalis, tricuspid annulus, or coronary sinus ostium. Left-sided atrial tachycardias often originate from around the pulmonary veins, atrial septum, or mitral annulus.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Atrial flutter
Organised re-entrant rhythm with atrial rates typically 250 to 350 bpm and ventricular rates often 145 to 150 bpm (due to 2:1 block) involving large areas of the atrium. In typical atrial flutter, the macroentry atrial circuit rotates around the tricuspid valve and between the inferior vena cava and tricuspid annulus. This essential part of the circuit can be a target for catheter ablation.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Atrial flutterFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].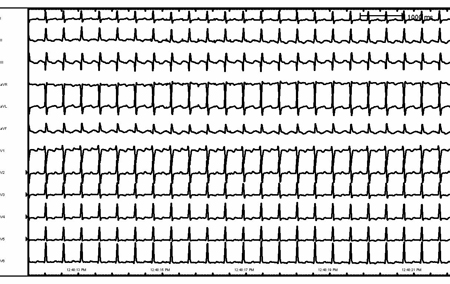 [Figure caption and citation for the preceding image starts]: Atrial flutter (detail)From the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Atrial flutter (detail)From the collection of Robert W. Rho, MD; used with permission [Citation ends].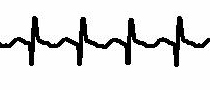
Sinus node re-entry tachycardia
A tachycardia that originates from re-entry circuits that involve the sinus node and perinodal tissue. It is presumed to be secondary to heterogeneous conduction properties of the sinus node and perinodal tissue.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
AV nodal re-entrant tachycardia (AVNRT)
A re-entrant tachycardia involving two pathways within the AV node or perinodal atrial tissue. One pathway has rapid conduction and a relatively long refractory period; the second pathway has slow conduction and a shorter refractory period. Following a premature atrial impulse, the fast pathway is still refractory from the previous impulse but anterograde conduction can occur through the slow pathway, which is no longer refractory. By the time slow-pathway conduction is complete, the fast pathway is no longer refractory and retrograde conduction can occur. If the slow pathway is no longer refractory following retrograde conduction, the cycle repeats. A less common form of AVNRT ('atypical' AVNRT) involves anterograde conduction down the fast pathway, resulting in a re-entry circuit that turns in the opposite direction to the more common 'typical' AVNRT described above.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
AV reciprocating tachycardia (AVRT)
A re-entrant tachycardia circuit that involves an accessory pathway as well as the native AV node. The most common form (approximately 90% of cases) is orthodromic AVRT.[25]Kotadia ID, Williams SE, O'Neill M. Supraventricular tachycardia: an overview of diagnosis and management. Clin Med (Lond). 2020 Jan;20(1):43-7.
https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/31941731
http://www.ncbi.nlm.nih.gov/pubmed/31941731?tool=bestpractice.com
The arrhythmia results from anterograde conduction through the AV node and retrograde conduction through the accessory pathway. This results in a narrow-complex tachycardia because the ventricle is activated anterograde through the His-Purkinje system.[26]Gillette PC, Garson AJ, Kugler JD. Wolff-Parkinson-White syndrome in children: electrophysiologic and pharmacologic characteristics. Circulation. 1979 Dec;60(7):1487-95.
http://circ.ahajournals.org/cgi/reprint/60/7/1487
http://www.ncbi.nlm.nih.gov/pubmed/498476?tool=bestpractice.com
Permanent junctional reciprocating tachycardia
A form of orthodromic AVRT involving anterograde conduction through the AV node and retrograde accessory-pathway conduction. The retrograde limb of this re-entry circuit is characterised by slow conduction, creating a very stable circuit (anterograde down the AV node and retrograde up the slowly conducting bypass tract). The descriptive term 'permanent' is added to reflect its stable nature and tendency to recur frequently and dominate sinus rhythm. For this reason, this arrhythmia can sometimes cause a tachycardia-mediated cardiomyopathy. This rhythm has rates between 120 and 200 bpm.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
It is most commonly seen in infants and children.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Junctional ectopic tachycardia
This rhythm is caused by abnormal rapid discharges from the junctional region (distal AV node or proximal His-Purkinje system) and does not require atrial or ventricular involvement to originate. The congenital form is insidious in presentation and is often identified in infants only after the development of tachycardia-induced cardiomyopathy. It is sometimes seen after cardiac surgery and may result in haemodynamic instability because of its rate and lack of AV synchrony. Clinical factors that may predispose to this arrhythmia include digitalis toxicity, hypokalaemia, myocardial ischaemia, cardiac surgery, and inflammatory myocarditis.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Narrow QRS (duration <120 ms) with an irregular ventricular rhythm
Atrial fibrillation
A supraventricular tachyarrhythmia characterised by an irregularly irregular rhythm due to the rapid and random conduction of irregular impulses to the ventricle. The irregular impulses are due to multiple wavelets of re-entry that rotate randomly within the atria and randomly bombard the AV node.[27]Veenhuyzen GD, Simpson CS, Abdollah H. Atrial fibrillation. CMAJ. 2004 Sep 28;171(7):755-60.
https://www.doi.org/10.1503/cmaj.1031364
http://www.ncbi.nlm.nih.gov/pubmed/15451840?tool=bestpractice.com
Risk of atrial fibrillation is increased in patients treated for hypertriglyceridaemia with medicine containing omega-3-acid ethyl esters, particularly at high doses.[28]Medicines and Healthcare products Regulatory Agency. Omega-3-acid ethyl ester medicines (Omacor/Teromeg 1000mg capsules): dose-dependent increased risk of atrial fibrillation in patients with established cardiovascular diseases or cardiovascular risk factors. Jan 2024 [internet publication].
https://www.gov.uk/drug-safety-update/omega-3-acid-ethyl-ester-medicines-omacor-slash-teromeg-1000mg-capsules-dose-dependent-increased-risk-of-atrial-fibrillation-in-patients-with-established-cardiovascular-diseases-or-cardiovascular-risk-factors
[29]Lombardi M, Carbone S, Del Buono MG, et al. Omega-3 fatty acids supplementation and risk of atrial fibrillation: an updated meta-analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother. 2021 Jul 23;7(4):e69-e70.
https://academic.oup.com/ehjcvp/article/7/4/e69/625523
[30]Gencer B, Djousse L, Al-Ramady OT, et al. Effect of long-term marine ɷ-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation. 2021 Dec 21;144(25):1981-90.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.121.055654
http://www.ncbi.nlm.nih.gov/pubmed/34612056?tool=bestpractice.com
Atrial tachycardia or flutter with variable AV conduction
In contrast to atrial fibrillation, rapid atrial tachycardia and atrial flutter result in fast but regular impulses to the AV node. Conduction of impulses to the ventricle is variable but tends to be 'regularly irregular' and with a pattern.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Multifocal atrial tachycardia
This arrhythmia involves at least three distinct competing atrial foci. Multifocal atrial tachycardia is most often associated with pulmonary disease but has also been associated with cardiac disease (valvular, hypertensive, coronary) as well as a variety of other systemic conditions, including hypokalaemia, hypomagnesaemia, and sepsis, and certain medications (including isoprenaline [isoproterenol] and aminophylline).[31]Shine KI, Kastor JA, Yurchak PM. Multifocal atrial tachycardia. Clinical and electrocardiographic features in 32 patients. N Engl J Med. 1968 Aug 15;279(7):344-9.
http://www.ncbi.nlm.nih.gov/pubmed/5662166?tool=bestpractice.com
[32]Iseri LT, Fairshter RD, Hardemann JL, et al. Magnesium and potassium therapy in multifocal atrial tachycardia. Am Heart J. 1985 Oct;110(4):789-94.
http://www.ncbi.nlm.nih.gov/pubmed/4050650?tool=bestpractice.com
Wide QRS (duration >120 ms) with a regular ventricular rhythm
Atrial tachycardia, atrial flutter, and common supraventricular tachycardias that conduct with aberrancy to the ventricle (left bundle branch block or right bundle branch block) are an important part of the differential diagnosis of aetiologies of wide-complex tachycardias with uniform QRS duration and morphology.
Idiopathic ventricular tachycardia (VT) (monomorphic VT associated with a structurally normal heart)
Repetitive monomorphic ventricular tachycardia: a focal arrhythmia that is thought to be due to triggered activity. Typically, it originates from the right ventricular outflow tract (when it is known as right ventricular outflow tract tachycardia). The aetiology is unknown. It is mostly seen in young and middle-aged patients of both sexes, with a structurally normal heart and is often provoked by exercise, emotion, stress, or hormone fluctuations.[33]Deal BJ, Miller SM, Scagliotti D, et al. Ventricular tachycardia in a young population without overt heart disease. Circulation. 1986 Jun;73(6):1111-8.
http://circ.ahajournals.org/cgi/reprint/73/6/1111
http://www.ncbi.nlm.nih.gov/pubmed/3698245?tool=bestpractice.com
Less commonly the site of origin may be in the LV outflow tract, the LV epicardium, above the pulmonary valve, or along the septal aspect of the mitral annulus. VT that maps to these regions behaves similarly to 'right ventricular outflow tract VT' and typically follows a benign clinical course.
Idiopathic left VT: occurs due to re-entry around the specialised conduction tissue and slow calcium-ion-sensitive myocardial tissue. The aetiology of this arrhythmia is unknown. It is sensitive to calcium channel blockers, which may terminate and control the arrhythmia. The re-entry circuit usually involves the left posterior fascicle and the VT is therefore relatively narrow, with a right bundle branch block and a superior axis on the 12-lead ECG. It is seen in patients aged 15 to 40 years old with structurally normal hearts. Seventy percent of cases are in males. In most cases, this VT is not associated with an elevated risk of sudden death.[34]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-391.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
[35]Zeppenfeld K, Tfelt-Hansen J, de Riva M, et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 26 Aug 2022 [Epub ahead of print].
https://academic.oup.com/eurheartj/advance-article/doi/10.1093/eurheartj/ehac262/6675633
http://www.ncbi.nlm.nih.gov/pubmed/36017572?tool=bestpractice.com
Accelerated idioventricular rhythm
An automatic focus originating in the ventricular myocardium. Similar to VT, though rates are no more than 20% faster than the sinus rate (typically 80-120 bpm).[34]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-391.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
Monomorphic VT associated with previous myocardial infarction
An arrhythmia that usually originates at the interface between healthy and damaged myocardium and is most commonly a re-entrant rhythm.[36]de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: electrophysiologic and anatomic correlation. Circulation. 1988 Mar;77(3):589-606.
http://circ.ahajournals.org/cgi/reprint/77/3/589
http://www.ncbi.nlm.nih.gov/pubmed/3342490?tool=bestpractice.com
It is more commonly seen with larger infarctions and in patients with a depressed ejection fraction.
Monomorphic VT associated with non-ischaemic cardiomyopathy
Regardless of the aetiology of the cardiomyopathy, heterogeneous conduction properties within the ventricular myocardium due to cardiomyopathy can serve as a substrate for re-entry and result clinically in VT. Some specific cardiomyopathies merit special attention.[34]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-391.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
Arrhythmogenic right ventricular dysplasia: characterised by fatty right ventricular infiltration and fibrosis or thinning. VT is due to re-entry within this complicated substrate.
Bundle branch re-entry VT: a common cause of sustained VT in patients with non-ischaemic dilated cardiomyopathy. The re-entry circuit usually conducts anterograde down the right bundle branch, across the ventricular septum, and conducts retrograde up the left bundle branch. The characteristic 12-lead ECG shows a left bundle branch block pattern during VT because of this pattern of activation.
Cardiac sarcoidosis: may present with arrhythmias (such as advanced AV block or VT) and/or unexplained new onset heart failure without a history of systemic sarcoidosis.[37]Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021 Oct;107(19):1591-9.[38]Nery PB, Mc Ardle BA, Redpath CJ, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2014 Mar;37(3):364-74.
http://www.ncbi.nlm.nih.gov/pubmed/24102263?tool=bestpractice.com
[39]Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014 Aug;25(8):875-81.
http://www.ncbi.nlm.nih.gov/pubmed/24602015?tool=bestpractice.com
The patchy formation of granulomas, fibrosis, and scarring leads to VT due to reentry within this complicated substrate.[37]Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021 Oct;107(19):1591-9. Clinically overt cardiac sarcoidosis has been reported in 5% to 10% of cases with systemic sarcoidosis.[37]Kouranos V, Sharma R. Cardiac sarcoidosis: state-of-the-art review. Heart. 2021 Oct;107(19):1591-9.[40]Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001 Nov 15;164(10 pt 1):1885-9.
https://www.atsjournals.org/doi/10.1164/ajrccm.164.10.2104046
http://www.ncbi.nlm.nih.gov/pubmed/11734441?tool=bestpractice.com
[41]Chow KL, O'Donnell JL, Crozier I. Prevalence, incidence and survival outcomes of cardiac sarcoidosis in the South Island, New Zealand. Int J Cardiol. 2022 Jun 15;357:128-33.
http://www.ncbi.nlm.nih.gov/pubmed/35395288?tool=bestpractice.com
Cardiac sarcoidosis has been confirmed in approximately one third of middle-aged patients (37%) presenting with unexplained high-grade atrioventricular block.[42]Maizels L, Mansour M, Abu-Much A, et al. Prevalence of cardiac sarcoidosis in middle-aged adults diagnosed with high-grade atrioventricular block. Am J Med. 2024 Apr;137(4):358-65.
http://www.ncbi.nlm.nih.gov/pubmed/38113953?tool=bestpractice.com
Chagas cardiomyopathy: VT is predominantly seen in chronic Chagas cardiomyopathy, which is probably caused by an autoimmune response to infection with Trypanosoma cruzi. The mechanism is likely to be related to the resulting fibrosis of the myocardium. The mechanism of VT is re-entry within this complicated substrate.
Rapid atrial arrhythmias associated with a 'bystander' accessory pathway
Any atrial arrhythmia (atrial flutter, supraventricular tachycardia ) that normally conducts through the native His-Purkinje system and would manifest with a narrow QRS can instead present as a wide-complex tachycardia if there is also an associated accessory pathway.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Additionally, with rapid supraventricular rhythms, a rate-related bundle branch may develop only at times of tachycardia, resulting in a wide-complex-tachycardia morphology.
Antidromic AV reciprocating tachycardia
Re-entrant circuit in which an anterograde circuit conducts down the accessory pathway and retrograde back up the AV node, resulting in a wide-QRS-complex morphology. The re-entry circuit in this arrhythmia is the same as that in orthodromic AV reciprocating tachycardia but in the opposite direction. Because activation is anterograde down the accessory pathway, the QRS complex is maximally pre-excited during tachycardia.[1]Page RL, Joglar JA, Caldwell MA, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2016 Apr 5;67(13):e27-115.
https://www.sciencedirect.com/science/article/pii/S0735109715058404?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/26409259?tool=bestpractice.com
[2]Katritsis DG, Boriani G, Cosio FG, et al. European Heart Rhythm Association (EHRA) consensus document on the management of supraventricular arrhythmias, endorsed by Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardiaca y Electrofisiologia (SOLAECE). Europace. 2017 Mar 1;19(3):465-511.
https://academic.oup.com/europace/article/19/3/465/2631183
http://www.ncbi.nlm.nih.gov/pubmed/27856540?tool=bestpractice.com
Paced rhythm
Patients with pacemakers can present with wide-complex tachycardia secondary to rapid ventricular pacing. Most commonly this is due to a dual chamber device tracking an atrial arrhythmia (atrial tachycardia and atrial flutter) with subsequent rapid ventricular pacing. [Figure caption and citation for the preceding image starts]: Right ventricular outflow tract ventricular tachycardiaFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].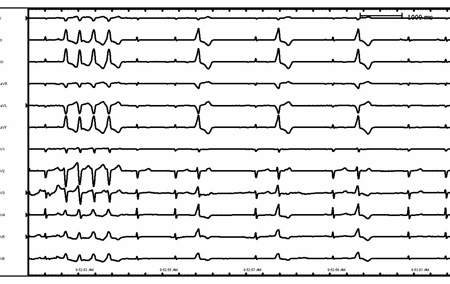 [Figure caption and citation for the preceding image starts]: Ventricular tachycardia in a patient with arrhythmogenic right ventricular cardiomyopathyFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ventricular tachycardia in a patient with arrhythmogenic right ventricular cardiomyopathyFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].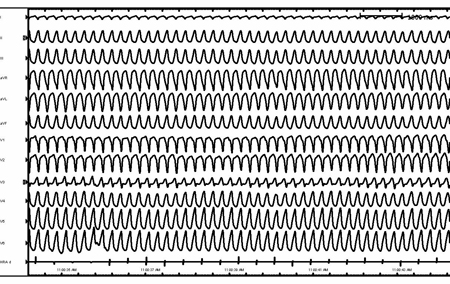 [Figure caption and citation for the preceding image starts]: Supraventricular tachycardia with aberrancy and left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Supraventricular tachycardia with aberrancy and left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].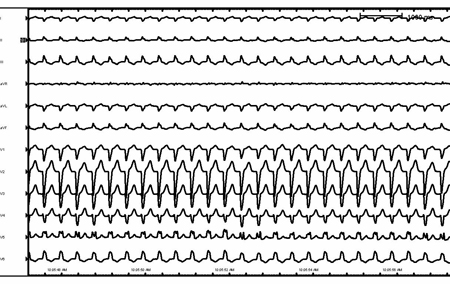 [Figure caption and citation for the preceding image starts]: Rate-related left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Rate-related left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].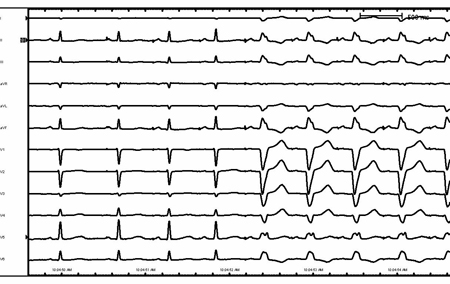 [Figure caption and citation for the preceding image starts]: Antidromic re-entrant tachycardiaFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Antidromic re-entrant tachycardiaFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].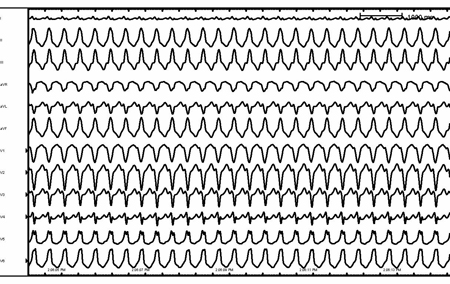
Wide QRS (duration >120 ms) with an irregular ventricular rhythm
Causes include the following:
Atrial fibrillation with a bundle branch block
Multifocal atrial tachycardia with a bundle branch block
Atrial tachycardia or flutter with variable AV conduction with a bundle branch block or accessory pathway.
Variable QRS duration with an irregular ventricular rhythm
Polymorphic VT with a normal QT interval
Most commonly seen in the context of acute coronary syndrome or myocardial ischaemia but can also be seen in structurally normal hearts. In the context of myocardial infarction, the development of polymorphic VT is suggestive of ongoing ischaemia; treatment is focused on the underlying ischaemia. Ion channelopathies can present with polymorphic VT. Catecholaminergic polymorphic VT occurs in the absence of structural heart disease and often presents as stress- or exercise-induced syncope, or sudden death in childhood or adolescence. A variety of genetic causes have been identified, one of which involves an autosomal dominant mutation in the gene for the cardiac ryanodine receptor.[43]Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001 Mar;85(2):321-41.
http://www.ncbi.nlm.nih.gov/pubmed/11233951?tool=bestpractice.com
Less commonly, an autosomal recessive mutation in the cardiac calsequestrin gene (CASQ2) has been associated with this condition. Both of these gene mutations are associated with the release of calcium from the sarcoplasmic reticulum.[44]The Cardiac Society of Australia and New Zealand. Guidelines for the diagnosis and management of catecholaminergic polymorphic ventricular tachycardia. Nov 2016 [internet publication].
https://www.csanz.edu.au/wp-content/uploads/2016/11/CPVT_Update_25-Nov-2016.pdf
Torsades de pointes
Polymorphic VT associated with a prolonged QT (observed during sinus rhythm) with a characteristic 'twisting on axis' morphology. The prolonged QT may be congenital or acquired. Acquired long-QT syndrome is more commonly observed clinically. It may be secondary to medications known to prolong the QT interval,
AZCERT: QT drugs list
Opens in new window ischaemia, significant electrolyte abnormalities (hypokalaemia, hypomagnesaemia, hypocalcaemia), or massive injury to the central nervous system.[43]Passman R, Kadish A. Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am. 2001 Mar;85(2):321-41.
http://www.ncbi.nlm.nih.gov/pubmed/11233951?tool=bestpractice.com
[45]Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020 Oct 13;142(15):e214-33.
https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000000905
http://www.ncbi.nlm.nih.gov/pubmed/32929996?tool=bestpractice.com
Bi-directional VT
A rare but potentially fatal arrhythmia most often caused by digitalis toxicity but also seen in catecholaminergic polymorphic VT and Andersen-Tawil syndrome (long QT 7). It is characterised by two alternating QRS morphologies (often a right bundle branch block with alternating left and right axis). Contrary to ventricular bigeminy, the R-R interval in bi-directional VT is regular.[34]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-391.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
Atrial fibrillation with ventricular pre-excitation
Patients with atrial fibrillation and an anterograde-conducting accessory pathway may present with a wide-complex irregular tachycardia. Some patients may have anterograde conduction pathways with more rapid properties than others and are therefore at greater risk of this arrhythmia. Identification of this arrhythmia is vital because it can lead to ventricular fibrillation and haemodynamic collapse. Pre-excited atrial fibrillation is a potentially life-threatening arrhythmia.[5]Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS guideline for the diagnosis and management of atrial fibrillation: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2024 Jan 2;149(1):e1-156.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11095842
http://www.ncbi.nlm.nih.gov/pubmed/38033089?tool=bestpractice.com
Ventricular fibrillation
Rapid (rate >300 bpm), grossly irregular, life-threatening ventricular rhythm characterised by variable QRS cycle length, morphology, and amplitude.[34]Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018 Sep 25;138(13):e272-391.
https://www.ahajournals.org/doi/10.1161/CIR.0000000000000549
http://www.ncbi.nlm.nih.gov/pubmed/29084731?tool=bestpractice.com
 [Figure caption and citation for the preceding image starts]: Atrial flutter (detail)From the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Atrial flutter (detail)From the collection of Robert W. Rho, MD; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Ventricular tachycardia in a patient with arrhythmogenic right ventricular cardiomyopathyFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Ventricular tachycardia in a patient with arrhythmogenic right ventricular cardiomyopathyFrom the collection of Robert W. Rho, MD; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Supraventricular tachycardia with aberrancy and left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Supraventricular tachycardia with aberrancy and left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Rate-related left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Rate-related left bundle branch blockFrom the collection of Robert W. Rho, MD; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Antidromic re-entrant tachycardiaFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Antidromic re-entrant tachycardiaFrom the collection of Robert W. Rho, MD; used with permission [Citation ends].