Acute respiratory distress syndrome (ARDS)
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
all patients
oxygen and ventilation
Although the original low tidal volume trial by the ARDS Network targeted an oxygen saturation between 88% and 95%, two subsequent clinical trials suggest that higher oxygenation targets may be associated with better clinical outcomes.[59]Barrot L, Asfar P, Mauny F, et al; LOCO2 Investigators and REVA Research Network. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020 Mar 12;382(11):999-1008. http://www.ncbi.nlm.nih.gov/pubmed/32160661?tool=bestpractice.com [61]Mackle D, Bellomo R, et al; ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020 Mar 12;382(11):989-98. http://www.ncbi.nlm.nih.gov/pubmed/31613432?tool=bestpractice.com Based on the findings of these studies, it seems prudent to target an oxygen saturation of ≥92%.[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927 http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com
With the increasing availability of high flow nasal oxygen (HFNO), the number of patients with ARDS who can be managed with either HFNO or non-invasive ventilation has increased. However, the failure rate is high and many patients with ARDS will require endotracheal intubation and mechanical ventilation.[63]Agarwal R, Aggarwal AN, Gupta D. Role of noninvasive ventilation in acute lung injury/acute respiratory distress syndrome: a proportion meta-analysis. Respir Care. 2010 Dec;55(12):1653-60. http://www.ncbi.nlm.nih.gov/pubmed/21122173?tool=bestpractice.com The American Thoracic Society (ATS) provides guidance on how to facilitate communication with mechanically ventilated patients as a key component of symptom assessment.[64]Guttormson JL, Khan B, Brodsky MB, et al. Symptom assessment for mechanically ventilated patients: principles and priorities: an official American Thoracic Society workshop report. Ann Am Thorac Soc. 2023 Apr;20(4):491-8. https://www.atsjournals.org/doi/10.1513/AnnalsATS.202301-023ST http://www.ncbi.nlm.nih.gov/pubmed/37000144?tool=bestpractice.com
Ventilator-associated lung injury may be limited by the use of a low tidal volume, plateau-pressure-limited protective ventilatory strategy. This therapy has been shown to reduce mortality.[65]Brower RG, Matthay MA, Morris A, et al; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. https://www.nejm.org/doi/10.1056/NEJM200005043421801 http://www.ncbi.nlm.nih.gov/pubmed/10793162?tool=bestpractice.com [66]Putensen C, Theuerkauf N, Zinserling J, et al. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009 Oct 20;151(8):566-76. http://www.ncbi.nlm.nih.gov/pubmed/19841457?tool=bestpractice.com [67]Walkey AJ, Goligher EC, Del Sorbo L, et al. Low tidal volume versus non-volume-limited strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017 Oct;14(suppl 4):S271-9. https://www.atsjournals.org/doi/full/10.1513/AnnalsATS.201704-337OT http://www.ncbi.nlm.nih.gov/pubmed/28846440?tool=bestpractice.com [68]American College of Emergency Physicians. Policy statement: mechanical ventilation. Oct 2017 [internet publication]. https://www.acep.org/patient-care/policy-statements/mechanical-ventilation
A tidal volume of 4-8 mL/kg predicted body weight should be used to maintain an inspiratory plateau pressure <30 cm H₂O with an initial setting of 6 mL/kg.[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com Predicted body weight for men is calculated as 50 + 0.91 × (height [cm] - 152.4), and for women is 45.5 + 0.91 × (height [cm] - 152.4).[65]Brower RG, Matthay MA, Morris A, et al; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. https://www.nejm.org/doi/10.1056/NEJM200005043421801 http://www.ncbi.nlm.nih.gov/pubmed/10793162?tool=bestpractice.com If the plateau pressure is >30 cm H₂O, then tidal volume should be lowered to 5 mL/kg or as low as 4 mL/kg, if needed.
Positive end-expiratory pressure (PEEP) and FiO₂ should be titrated using established PEEP titration tables.[65]Brower RG, Matthay MA, Morris A, et al; Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1301-8. https://www.nejm.org/doi/10.1056/NEJM200005043421801 http://www.ncbi.nlm.nih.gov/pubmed/10793162?tool=bestpractice.com [70]Brower RG, Lanken PN, MacIntyre N, et al; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004 Jul 22;351(4):327-36. https://www.nejm.org/doi/full/10.1056/NEJMoa032193 http://www.ncbi.nlm.nih.gov/pubmed/15269312?tool=bestpractice.com The available data suggest that higher levels of PEEP are safe and may improve oxygenation in some patients.[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com [71]Meade MO, Cook DJ, Guyatt GH, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):637-45. https://jamanetwork.com/journals/jama/fullarticle/181425 http://www.ncbi.nlm.nih.gov/pubmed/18270352?tool=bestpractice.com [72]Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008 Feb 13;299(6):646-55. https://jamanetwork.com/journals/jama/fullarticle/181426 http://www.ncbi.nlm.nih.gov/pubmed/18270353?tool=bestpractice.com In a meta-analysis of available trials, there was no overall reduction in mortality with higher PEEP.[73]Santa Cruz R, Villarejo F, Irrazabal C, et al. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2021 Mar 30;3(3):CD009098. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8094163 http://www.ncbi.nlm.nih.gov/pubmed/33784416?tool=bestpractice.com An earlier meta-analysis suggested that higher PEEP reduces mortality in patients who respond with improved oxygenation.[74]Guo L, Xie J, Huang Y, et al. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: a systematic review and meta-analysis. BMC Anesthesiol. 2018 Nov 17;18(1):172. https://bmcanesthesiol.biomedcentral.com/articles/10.1186/s12871-018-0631-4 http://www.ncbi.nlm.nih.gov/pubmed/30447683?tool=bestpractice.com
Respiratory acidosis, a common complication of low tidal volume ventilation, is treated by increasing the respiratory rate. Although it is not known what level of respiratory acidosis is harmful in patients with ARDS, permissive hypercapnia is often tolerated due to low tidal volume ventilation. However, severe hypercapnia is independently associated with higher intensive care unit mortality.[79]Nin N, Muriel A, Peñuelas O, et al; VENTILA Group. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017 Feb;43(2):200-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5630225 http://www.ncbi.nlm.nih.gov/pubmed/28108768?tool=bestpractice.com Normocapnia often cannot be achieved (and should not be a goal). Clinical guidelines recommend that an arterial pH of 7.30 to 7.45 is maintained, but studies suggest patients who undergo permissive hypercapnia can tolerate a blood pH as low as 7.15. Bicarbonate infusions may be administered when the pH falls below 7.15.
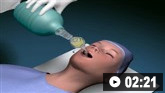
How to insert a tracheal tube in an adult using a laryngoscope.
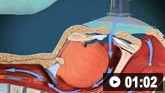
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
Selected patients with COVID-19 and mild ARDS can be considered for a trial of high-flow nasal oxygen or non-invasive ventilation. Endotracheal intubation should be not delayed if there is no improvement after a short trial (1 hour).[128]World Health Organization. COVID-19 clinical management: living guidance. January 2021 [internet publication]. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
See our topic Coronavirus disease 2019 (COVID-19) for more information on the management of COVID-19.
prone positioning
Additional treatment recommended for SOME patients in selected patient group
Prone positioning can improve oxygenation in patients with ARDS and has been shown to reduce mortality in patients with severe ARDS (PaO₂/fraction of inspired oxygen [FiO₂] <150).[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com [83]Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med. 2010 Apr;36(4):585-99. http://www.ncbi.nlm.nih.gov/pubmed/20130832?tool=bestpractice.com [84]Abroug F, Ouanes-Besbes L, Dachraoui F, et al. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care. 2011;15(1):R6. https://ccforum.biomedcentral.com/articles/10.1186/cc9403 http://www.ncbi.nlm.nih.gov/pubmed/21211010?tool=bestpractice.com [85]Bloomfield R, Noble DW, Sudlow A. Prone position for acute respiratory failure in adults. Cochrane Database Syst Rev. 2015 Nov 13;(11):CD008095. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008095.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/26561745?tool=bestpractice.com [86]Guérin C, Reignier J, Richard JC, et al; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013 Jun 6;368(23):2159-68. http://www.ncbi.nlm.nih.gov/pubmed/23688302?tool=bestpractice.com [87]Beitler JR, Shaefi S, Montesi SB, et al. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014 Mar;40(3):332-41. http://www.ncbi.nlm.nih.gov/pubmed/24435203?tool=bestpractice.com One systematic review found that reduced mortality was contingent upon patients remaining prone for at least 12 hours daily.[88]Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017 Oct;14(suppl 4):S280-8. https://www.atsjournals.org/doi/full/10.1513/AnnalsATS.201704-343OT http://www.ncbi.nlm.nih.gov/pubmed/29068269?tool=bestpractice.com Given the potential complications of prone positioning, including facial oedema, pressure sores, and dislodgement of catheters and endotracheal tubes, prone positioning should only be considered in patients with severe ARDS (PaO₂/FiO₂ <150).[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com
Prone positioning is recommended for patients with COVID-19 and severe ARDS (12-16 hours per day). Awake prone positioning can be considered for patients with COVID-19 receiving high-flow nasal oxygen or non-invasive ventilation.[128]World Health Organization. COVID-19 clinical management: living guidance. January 2021 [internet publication]. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 [131]Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021 Mar 1;49(3):e219-34. https://journals.lww.com/ccmjournal/Fulltext/2021/03000/Surviving_Sepsis_Campaign_Guidelines_on_the.21.aspx http://www.ncbi.nlm.nih.gov/pubmed/33555780?tool=bestpractice.com
See our topic Coronavirus disease 2019 (COVID-19) for more information on the management of COVID-19.
intravenous fluids
Additional treatment recommended for SOME patients in selected patient group
The patient's fluid balance should be maintained as slightly negative or neutral (providing the patient is not in shock).[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927 http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com A central line is recommended to measure the central venous pressure (CVP), with regular assessments of fluid status. The goal is to keep the CVP <4 cm H₂O. The routine use of a pulmonary artery catheter (to measure pulmonary artery occlusion pressure) is not recommended as insertion is associated with more complications than a central line.[47]National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Network. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006 May 25;354(21):2213-24. http://www.ncbi.nlm.nih.gov/pubmed/16714768?tool=bestpractice.com
A conservative fluid strategy reduced the duration of mechanical ventilation but had no effect on mortality in a large clinical trial in patients with ARDS who were not in shock.[89]Wiedemann HP, Wheeler AP, Bernard GR, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564-75. http://www.ncbi.nlm.nih.gov/pubmed/16714767?tool=bestpractice.com Similar results were reported in one systematic review and meta-analysis of adults and children with ARDS, sepsis, or systemic inflammatory response syndrome.[90]Silversides JA, Major E, Ferguson AJ, et al. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive Care Med. 2017 Feb;43(2):155-70. http://www.ncbi.nlm.nih.gov/pubmed/27734109?tool=bestpractice.com
antimicrobials + identification and treatment of source of infection
Additional treatment recommended for SOME patients in selected patient group
In patients who have an infectious cause for ARDS (e.g., pneumonia or sepsis), the prompt initiation of antimicrobials is important.[101]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67. https://www.atsjournals.org/doi/full/10.1164/rccm.201908-1581ST http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com [102]Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016 Sep 1;63(5):e61-111. https://academic.oup.com/cid/article/63/5/e61/2237650 http://www.ncbi.nlm.nih.gov/pubmed/27418577?tool=bestpractice.com Empirical antibiotics targeted at the suspected underlying infection should be used as soon as possible after obtaining appropriate cultures including blood, sputum, and urine cultures. Antivirals or antifungals may be appropriate in patients with suspected or confirmed viral or fungal infections. Once culture results are available, the antimicrobial regimen can be tailored for the identified organism. There is no evidence to support the use of antibiotics in patients who have ARDS without infection.
There are conflicting recommendations across international guidelines about the use of the antiviral remdesivir in patients with COVID-19. Local guidance and protocols should be consulted.
Patients with COVID-19 should be managed with appropriate isolation and infection prevention and control measures.
There is a strong recommendation that patients with ARDS due to COVID-19 should be treated with Il-6 inhibitors (tocilizumab or sarilumab) and the Janus Kinase (JAK) inhibitor baricitinib.[139]World Health Organization. Therapeutics and COVID-19: living guideline. Nov 2023 [internet publication]. https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.2 BMJ: a living WHO guideline on drugs for Covid-19 Opens in new window
See our topic Coronavirus disease 2019 (COVID-19) for more information on the management of COVID-19.
supportive care
Additional treatment recommended for SOME patients in selected patient group
Standard supportive care of critically ill patients includes prevention of deep vein thrombosis, blood glucose control, prophylaxis against stress-induced gastrointestinal bleeding, haemodynamic support to maintain a mean arterial pressure >60 mmHg, and transfusion of packed red blood cells in patients with haemoglobin <70 g/L (<7 g/dL).[103]Samama MM, Cohen AT, Darmon JY, et al; Prophylaxis in Medical Patients with Enoxaparin Study Group. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med. 1999 Sep 9;341(11):793-800. https://www.nejm.org/doi/10.1056/NEJM199909093411103 http://www.ncbi.nlm.nih.gov/pubmed/10477777?tool=bestpractice.com [104]Cook D, Guyatt G, Marshall J, et al; Canadian Critical Care Trials Group. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. N Engl J Med. 1998 Mar 19;338(12):791-7. https://www.nejm.org/doi/full/10.1056/NEJM199803193381203 http://www.ncbi.nlm.nih.gov/pubmed/9504939?tool=bestpractice.com Nutrition should be provided enterally where possible.[105]Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001 Dec;29(12):2264-70. http://www.ncbi.nlm.nih.gov/pubmed/11801821?tool=bestpractice.com In one large randomised trial of 1000 patients with ARDS, low-dose enteral feeding for the first 5 days of ARDS had similar clinical outcomes compared with full-calorie feeding.[106]Rice TW, Wheeler AP, Thompson BT, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012 Feb 22;307(8):795-803. https://jamanetwork.com/journals/jama/fullarticle/1355969 http://www.ncbi.nlm.nih.gov/pubmed/22307571?tool=bestpractice.com Supplemental nutrition with omega-3 fatty acids and antioxidants is not recommended.[107]Dushianthan A, Cusack R, Burgess VA, et al. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev. 2019 Jan 24;(1):CD012041. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012041.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/30677127?tool=bestpractice.com
Inhaled or intravenous beta-adrenergic agonists to promote alveolar fluid clearance and resolution of pulmonary oedema are not recommended.[108]Matthay MA, Brower RG, Carson S, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized beta2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011 Sep 1;184(5):561-8. https://www.atsjournals.org/doi/full/10.1164/rccm.201012-2090OC http://www.ncbi.nlm.nih.gov/pubmed/21562125?tool=bestpractice.com [109]Gao Smith F, Perkins GD, Gates S, et al; BALTI-2 study investigators. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012 Jan 21;379(9812):229-35. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)61623-1/fulltext http://www.ncbi.nlm.nih.gov/pubmed/22166903?tool=bestpractice.com Neither early nor late administration of corticosteroids has been shown to improve mortality in patients with ARDS, and their routine use is not recommended in patients who do not have COVID-19.[110]Bernard GR, Luce JM, Sprung CL. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987 Dec 17;317(25):1565-70. http://www.ncbi.nlm.nih.gov/pubmed/3317054?tool=bestpractice.com [111]Steinberg KP, Hudson LD, Goodman RB, et al; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006 Apr 20;354(16):1671-84. http://www.ncbi.nlm.nih.gov/pubmed/16625008?tool=bestpractice.com
Corticosteroids (low-dose intravenous or oral dexamethasone or an alternative corticosteroid) are strongly recommended for adults with severe or critical COVID-19 disease, including those with ARDS, based on several large randomised clinical trials. The recommended duration of treatment is 7 to 10 days.[129]WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020 Oct 6;324(13):1330-41. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7489434 http://www.ncbi.nlm.nih.gov/pubmed/32876694?tool=bestpractice.com [130]Agarwal A, Hunt B, Stegemann M, et al. A living WHO guideline on drugs for covid-19. BMJ. 2022 Apr 25;377:o1045. https://www.bmj.com/content/370/bmj.m3379.long
See our topic Coronavirus disease 2019 (COVID-19) for more information on the management of COVID-19.
rescue therapies
Additional treatment recommended for SOME patients in selected patient group
In patients with refractory hypoxaemia despite a fraction of inspired oxygen (FiO₂) of 1.0 and high levels of positive end-expiratory pressure (PEEP), rescue therapies for oxygenation should be considered.[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927 http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com
Neuromuscular paralysis improves ventilator-patient synchrony and often improves oxygenation. Intermittent doses of paralytics can be used as effectively as a continuous intravenous infusion. If a patient is on a continuous intravenous infusion of a paralytic, train-of-four monitoring should be used to monitor the muscle fibre twitch response to the drug. Given findings from randomised controlled trials, neuromuscular blockade should be reserved for patients with severe ARDS and refractory hypoxaemia despite low tidal volume ventilation and adequate sedation, particularly if there is still evidence of ventilator-patient dyssynchrony.[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927 http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com [112]Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010 Sep 16;363(12):1107-16. http://www.ncbi.nlm.nih.gov/pubmed/20843245?tool=bestpractice.com [113]Moss M, Huang DT, Brower RG, et al; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019 May 23;380(21):1997-2008. https://www.nejm.org/doi/full/10.1056/NEJMoa1901686 http://www.ncbi.nlm.nih.gov/pubmed/31112383?tool=bestpractice.com The ATS suggests using neuromuscular blockers in patients with early severe ARDS.[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com
Inhaled nitric oxide can improve oxygenation in patients with ARDS, but does not improve mortality and has been associated with acute kidney injury.[114]Taylor RW, Zimmerman JL, Dellinger RP, et al; Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA. 2004 Apr 7;291(13):1603-9.
http://www.ncbi.nlm.nih.gov/pubmed/15069048?tool=bestpractice.com
[115]Adhikari NK, Burns KE, Friedrich JO, et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ. 2007 Apr 14;334(7597):779.
https://www.bmj.com/content/334/7597/779.long
http://www.ncbi.nlm.nih.gov/pubmed/17383982?tool=bestpractice.com
[116]Gebistorf F, Karam O, Wetterslev J, et al. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults. Cochrane Database Syst Rev. 2016 Jun 27;(6):CD002787.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002787.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/27347773?tool=bestpractice.com
[  ]
What are the benefits and harms of inhaled nitric oxide in children and adults with acute respiratory distress syndrome?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1376/fullShow me the answer Thus, it should be used only as a rescue therapy for refractory hypoxaemia.[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927
http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com
Inhaled prostacyclin is easier to administer than inhaled nitric oxide, and also has the potential to improve oxygenation in ARDS through better ventilation perfusion matching. However, there are currently no published large randomised controlled trials of inhaled prostacyclin; thus, it should be used cautiously and only as a rescue therapy.[117]Afshari A, Bastholm Bille A, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2017 Jul 24;(7):CD007733.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007733.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28806480?tool=bestpractice.com
]
What are the benefits and harms of inhaled nitric oxide in children and adults with acute respiratory distress syndrome?/cca.html?targetUrl=https://cochranelibrary.com/cca/doi/10.1002/cca.1376/fullShow me the answer Thus, it should be used only as a rescue therapy for refractory hypoxaemia.[62]Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021 Aug 14;398(10300):622-37.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248927
http://www.ncbi.nlm.nih.gov/pubmed/34217425?tool=bestpractice.com
Inhaled prostacyclin is easier to administer than inhaled nitric oxide, and also has the potential to improve oxygenation in ARDS through better ventilation perfusion matching. However, there are currently no published large randomised controlled trials of inhaled prostacyclin; thus, it should be used cautiously and only as a rescue therapy.[117]Afshari A, Bastholm Bille A, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2017 Jul 24;(7):CD007733.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007733.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28806480?tool=bestpractice.com
Where available, extracorporeal membrane oxygenation (ECMO) should be considered (in conjunction with low tidal volume mechanical ventilation) in select patients with severe ARDS in whom standard therapies are failing (i.e., patients with profound refractory hypoxaemia).[69]Qadir N, Sahetya S, Munshi L, et al. An update on management of adult patients with acute respiratory distress syndrome: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2024 Jan 1;209(1):24-36. https://www.atsjournals.org/doi/10.1164/rccm.202311-2011ST http://www.ncbi.nlm.nih.gov/pubmed/38032683?tool=bestpractice.com [118]Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019 May 24;6(1):e000420. https://bmjopenrespres.bmj.com/content/6/1/e000420.info http://www.ncbi.nlm.nih.gov/pubmed/31258917?tool=bestpractice.com One multi-centre trial showed that patients with severe ARDS randomised to transfer to a tertiary care center for consideration of ECMO (75% [n=68] of whom actually received ECMO) were more likely to survive to 6 months without disability than patients randomised to continued conventional management (RR 0.69, 95% CI 0.05 to 0.97, P=0.03).[119]Peek GJ, Mugford M, Tiruvoipati R, et al; CESAR Trial Collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009 Oct 17;374(9698):1351-63. http://www.ncbi.nlm.nih.gov/pubmed/19762075?tool=bestpractice.com One subsequent randomised multi-centre trial (n=249) did not demonstrate significantly lower 60-day mortality in the ECMO treatment group compared with standard care (35% vs. 46%, respectively; P=0.09); however, one meta-analysis pooling data from both trials reported significantly lower 60-day mortality in the venovenous ECMO group compared with the control group (RR 0.73, 95% CI 0.58 to 0.92, P=0.008) despite a moderate risk of major bleeding in the ECMO group.[120]Combes A, Hajage D, Capellier G, et al; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018 May 24;378(21):1965-75. https://www.nejm.org/doi/10.1056/NEJMoa1800385 http://www.ncbi.nlm.nih.gov/pubmed/29791822?tool=bestpractice.com [121]Munshi L, Walkey A, Goligher E, et al. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019 Feb;7(2):163-72. http://www.ncbi.nlm.nih.gov/pubmed/30642776?tool=bestpractice.com An additional meta-analysis that included trials in critically ill patients with indications other than ARDS found that ECMO was associated with a reduction in day‐90 to one‐year all‐cause mortality, along with a threefold increased risk of bleeding.[122]Burrell A, Kim J, Alliegro P, et al. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2023 Sep 26;9(9):CD010381. http://www.ncbi.nlm.nih.gov/pubmed/37750499?tool=bestpractice.com
Routine use of high-frequency oscillatory ventilation (HFOV) in moderate-to-severe ARDS is not beneficial, and may be harmful.[123]Young D, Lamb SE, Shah S, et al; OSCAR Study Group. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013 Feb 28;368(9):806-13. http://www.ncbi.nlm.nih.gov/pubmed/23339638?tool=bestpractice.com [124]Sud S, Sud M, Friedrich JO, et al. High-frequency oscillatory ventilation versus conventional ventilation for acute respiratory distress syndrome. Cochrane Database Syst Rev. 2016 Apr 4;(4):CD004085. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD004085.pub4/full http://www.ncbi.nlm.nih.gov/pubmed/27043185?tool=bestpractice.com [125]Goligher EC, Munshi L, Adhikari NKJ, et al. High-frequency oscillation for adult patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc. 2017 Oct;14(suppl 4):S289-96. https://www.atsjournals.org/doi/full/10.1513/AnnalsATS.201704-341OT http://www.ncbi.nlm.nih.gov/pubmed/29043832?tool=bestpractice.com [126]Ferguson ND, Cook DJ, Guyatt GH, et al; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013 Feb 28;368(9):795-805. http://www.ncbi.nlm.nih.gov/pubmed/23339639?tool=bestpractice.com [127]Meade MO, Young D, Hanna S, et al. Severity of hypoxemia and effect of high-frequency oscillatory ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017 Sep 15;196(6):727-33. https://www.atsjournals.org/doi/full/10.1164/rccm.201609-1938OC http://www.ncbi.nlm.nih.gov/pubmed/28245137?tool=bestpractice.com

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer