Suspect sarcopenia in a patient older than 65 years who presents with signs and symptoms such as falling, feeling weak, walking slowly, difficulty rising from a chair, and weight/muscle loss. Several clinical criteria for sarcopenia have been proposed as part of the four main international definitions, with some variations in the selected criteria and cut-offs.
The European Working Group for Sarcopenia in Older People 2 (EWGSOP2) recommends using the SARC-F patient questionnaire to identify people with suspected sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[26]Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016 Mar;7(1):28-36.
https://onlinelibrary.wiley.com/doi/10.1002/jcsm.12048
http://www.ncbi.nlm.nih.gov/pubmed/27066316?tool=bestpractice.com
The International Conference on Sarcopenia and Frailty Research (ICSFR) working group recommends using gait speed or SARC-F for initial screening.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
If the SARC-F score is ≥4 or if the usual gait speed is low (i.e., a risk of sarcopenia is present), further assessment for muscle strength, quality, and quantity and functional performance is warranted.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
A calf circumference measurement can be added to the SARC-F questionnaire (SARC-CalF). The Asian Working Group for Sarcopenia (AWGS) proposes separate algorithms for community and hospital settings, which both begin by screening either calf circumference (<34 cm in men and <33 cm in women), SARC-F (≥4), or SARC-CalF (≥11) as a method of identifying people at risk for sarcopenia.[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
The Sarcopenia Definition and Outcomes Consortium (SDOC) proposes grip strength, either absolute or scaled to measures of body size, and gait speed as the only parameters to be considered in the diagnosis of sarcopenia.[3]Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020 Jul;68(7):1410-8.
http://www.ncbi.nlm.nih.gov/pubmed/32150289?tool=bestpractice.com
[27]Kirk B, Zanker J, Bani Hassan E, et al. Sarcopenia Definitions and Outcomes Consortium (SDOC) criteria are strongly associated with malnutrition, depression, falls, and fractures in high-risk older persons. J Am Med Dir Assoc. 2021 Apr;22(4):741-5.
http://www.ncbi.nlm.nih.gov/pubmed/32771358?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: EWGSOP2 algorithm for case-finding, making a diagnosis and quantifying severity of sarcopenia in practice. EWGSOP2: European Working Group for Sarcopenia in Older People 2; SARC-F: Strength, Ambulation, Rising from a chair, Climbing stairs, Falls; DXA: Dual-energy x-ray absorptiometry; BIA: Bioimpedance analysis; SPPB: Short Physical Performance Battery; TUG: Timed up-and-go testCruz-Jentoft AJ et al; Age Ageing. 2019 Jan 1;48(1):16-31 [Citation ends].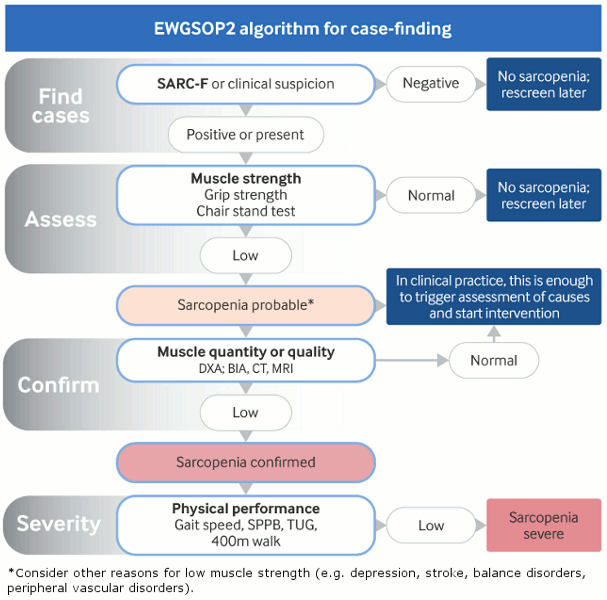
Consider low muscle strength (measured by hand grip test or sit-stand [chair raise] test) as the primary parameter of sarcopenia in patients aged 65 years and over. Low muscle strength alone can be enough to diagnose probable sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
Based on the EWGSOP2 guidelines, the presence of low muscle quantity or quality (measured by imaging; criteria vary) confirms the diagnosis. Consider sarcopenia to be severe if low physical performance (measured by gait speed) is detected in addition to low muscle strength and low muscle quantity or quality.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
History
Gathering a pertinent history is an important component of the assessment of patients with probable sarcopenia. EWGSOP2 considers low muscle strength to be the primary indicator of probable sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
Also consider any of the following as possible indications of sarcopenia:[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[28]Morley JE. Sarcopenia in the elderly. Fam Pract. 2012 Apr;29(suppl 1):i44-8.
https://academic.oup.com/fampra/article/29/suppl_1/i44/531163
http://www.ncbi.nlm.nih.gov/pubmed/22399555?tool=bestpractice.com
History of weight loss
Slow usual gait speed
Difficulty in performing activities of daily living (cleaning, shopping for groceries)
Difficulty with activities related to mobility (walking, climbing stairs, taking public transport)
Falls
Fear of falling
Reduction in outdoor activities or being housebound.
Ask about risk factors for the development of sarcopenia, such as sedentary lifestyle, smoking, and low protein intake. Patients with sarcopenia are not necessarily aware of the decrease of muscle mass and strength, and the development of sarcopenia.
Ask about the presence of comorbidities, such as chronic obstructive pulmonary disease and chronic heart or kidney failure, which contribute to weakness and deterioration of physical performance.[15]Pacifico J, Geerlings MAJ, Reijnierse EM, et al. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta-analysis. Exp Gerontol. 2020 Mar;131:110801.
https://www.sciencedirect.com/science/article/pii/S0531556519307417
http://www.ncbi.nlm.nih.gov/pubmed/31887347?tool=bestpractice.com
Also search for other causes of muscle weakness or slow gait speed (e.g., Parkinson's disease or other neurological disease). Consider the speed of onset of muscle weakness and the pattern of muscle weakness when considering other causes.
Review any medications the patient is taking. Some medications contribute to a suppressed appetite and some are associated with muscle loss, such as corticosteroids, gonadotrophin-releasing hormone (GnRH) agonists, anti-testosterone agents, and meglitinide (glinide) antidiabetic medications.
SARC-F questionnaire
SARC-F is a screening questionnaire.[26]Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016 Mar;7(1):28-36.
https://onlinelibrary.wiley.com/doi/10.1002/jcsm.12048
http://www.ncbi.nlm.nih.gov/pubmed/27066316?tool=bestpractice.com
It is considered a practical tool to initiate screening and is appropriate in community healthcare or other clinical settings.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
It addresses:
Strength
Ambulation (walking)
Rising from a chair
Climbing stairs
Falls.
Each of the five items has a minimum of 0 and a maximum of 2 points for a maximum score of 10. A total score ≥4 indicates that the patient is at risk of sarcopenia.
European (EWGSOP2) and international (ICSFR) guidelines recommended using SARC-F as a screening tool for sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
It has good correlation and specificity with muscle function tests and seems useful for identifying muscle function deficits, muscle atrophy, and low strength.[29]Li M, Kong Y, Chen H, et al Accuracy and prognostic ability of the SARC-F questionnaire and Ishii's score in the screening of sarcopenia in geriatric inpatients. Braz J Med Biol Res. 2019;52(9):e8204.
https://www.scielo.br/j/bjmbr/a/5q5dTqWcXRpf3VvFT5mpGzx/?lang=en
http://www.ncbi.nlm.nih.gov/pubmed/31482974?tool=bestpractice.com
A calf circumference measurement can be added to the SARC-F questionnaire (SARC-CalF). The AWGS proposes separate algorithms for community and hospital settings, which both begin by screening either calf circumference (<34 cm in men and <33 cm in women), SARC-F (≥4), or SARC-CalF (≥11) as a method of identifying people at risk for sarcopenia.[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
The sensitivity of the SARC-CalF is still debatable since some researchers show that adding the calf circumference does not improve the sensitivity for the screening of sarcopenia.[30]Bahat G, Erdoğan T, İlhan B. SARC-F and other screening tests for sarcopenia. Curr Opin Clin Nutr Metab Care. 2022 Jan 1;25(1):37-42.
http://www.ncbi.nlm.nih.gov/pubmed/34861669?tool=bestpractice.com
However, it is an accessible and acceptable measure.
The Ishii tool is another practical way of assessing sarcopenia. In the Ishii screening test, a score evaluating the probability of sarcopenia is calculated using age, grip strength, and calf circumference.[31]Ishii S, Tanaka T, Shibasaki K, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014 Feb;14(suppl 1):93-101.
https://onlinelibrary.wiley.com/doi/10.1111/ggi.12197
http://www.ncbi.nlm.nih.gov/pubmed/24450566?tool=bestpractice.com
Physical examination
The main considerations in a physical examination are:
Excessive emaciation or adiposity
Abnormal anthropometric measurements
Low hand grip strength
Difficulty rising from a chair
Slowness of movement (i.e. slow gait speed).
Look for excessive emaciation or adiposity. Even with obesity, some people have a relative loss of muscle mass.[24]DeMik DE, Marinier MC, Glass NA, et al. Prevalence of sarcopenia and sarcopenic obesity in an academic total joint arthroplasty practice. Arthroplast Today. 2022 Jun 4;16:124-9.
https://www.arthroplastytoday.org/article/S2352-3441(22)00114-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35677943?tool=bestpractice.com
Use objective anthropometric measurements. Body mass index (BMI) is an anthropometric measurement based on weight (in kg) divided by height (in m) squared (kg/m²). It is used to diagnose sarcopenic obesity as it is an easy-to-perform estimation of adiposity as well as of cardiovascular risk. By the World Health Organization definition, any value ≥30 kg/m² is diagnostic of obesity and a BMI of below 18.5 is considered underweight.[10]Global recommendations on physical activity for health. Geneva: World Health Organization; 2010.
https://www.ncbi.nlm.nih.gov/books/NBK305057
http://www.ncbi.nlm.nih.gov/pubmed/26180873?tool=bestpractice.com
When addressing physical function, consider the negative impact of obesity, especially in relation to mobility.[32]Holmgren M, Lindgren A, de Munter J, et al. Impacts of mobility disability and high and increasing body mass index on health-related quality of life and participation in society: a population-based cohort study from Sweden. BMC Public Health. 2014 Apr 17;14:381.
https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-14-381
http://www.ncbi.nlm.nih.gov/pubmed/24742257?tool=bestpractice.com
Patients with low muscle mass and strength but high adiposity may have sarcopenic obesity syndrome.[33]Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004 Dec;12(12):1995-2004.
https://onlinelibrary.wiley.com/doi/10.1038/oby.2004.250
http://www.ncbi.nlm.nih.gov/pubmed/15687401?tool=bestpractice.com
There is not yet a consensus for the diagnostic criteria for this syndrome and its prevalence is estimated to be much lower than sarcopenia alone (excess weight is a form of resistance exercise minimising muscle loss). Regardless, sarcopenic obesity carries a greater risk of disability than sarcopenia alone.[34]Barazzoni R, Bischoff S, Boirie Y, et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018 Jul 18;11(4):294-305.
https://karger.com/ofa/article/11/4/294/239582/Sarcopenic-Obesity-Time-to-Meet-the-Challenge
http://www.ncbi.nlm.nih.gov/pubmed/30016792?tool=bestpractice.com
Observe in the course of the examination how the patient stands from a chair and mobilises. Perform a neurological examination. This should focus on the peripheral musculoskeletal system to evaluate, in particular, muscle bulk and strength of the different body segments. Search for other causes of muscle weakness or slow gait speed (e.g., Parkinson's disease or other neurological disease). Consider the speed of onset of muscle weakness and the pattern of muscle weakness when considering other causes.
Hand grip strength (HGS)
Use HGS to determine low levels of muscle function when diagnosing sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
The International Clinical Practice guidelines for Sarcopenia state that cut-off values for HGS should be tailored to the population.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
EWGSOP2 suggests measuring HGS as the primary parameter of sarcopenia, because muscle strength is the most reliable measure of muscle function.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
The threshold used by EWGSOP2 for low HGS is <27 kg for men and <16 kg for women, based on normative data from 12 British studies.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[35]Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014 Dec 4;9(12):e113637.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0113637
http://www.ncbi.nlm.nih.gov/pubmed/25474696?tool=bestpractice.com
This equated to 23.0% of men and 26.6% of women at age 80 as having weak HGS in the study population.[35]Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014 Dec 4;9(12):e113637.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0113637
http://www.ncbi.nlm.nih.gov/pubmed/25474696?tool=bestpractice.com
AWGS suggested a similar approach to the EWGSOP2 based on available evidence in Asia.[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
They recommended using both HGS and gait speed as primary screening tests. The Asian working group's cut-off values regarding HGS differed based on the specific populations studied.[36]Wu SW, Wu SF, Liang HW, et al. Measuring factors affecting grip strength in a Taiwan Chinese population and a comparison with consolidated norms. Appl Ergon. 2009 Jul;40(4):811-5.
http://www.ncbi.nlm.nih.gov/pubmed/18947819?tool=bestpractice.com
The study included 482 participants from Taiwan and compared HGS with consolidated results acquired from a mainly white population. The mean HGS was 25% lower in men and 27% in women in the sample population from Taiwan. Following several studies and comparisons between different Asian populations, AWGS defined low HGS as <28 kg for men and <18 kg for women based on the lower 20th percentile of HGS in these studies.[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
[37]Tanimoto Y, Watanabe M, Sun W, et al. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch Gerontol Geriatr. 2012 Sep-Oct;55(2):e9-13.
http://www.ncbi.nlm.nih.gov/pubmed/22795189?tool=bestpractice.com
Sit-stand test
The sit-stand test (also called the chair rise test or chair stand test) is a lower extremity strength test that can be used as a proxy for strength measurements. It focuses on the lower extremities (quadriceps muscles), and the only equipment required to perform this measurement is a chair. The sit-stand test measures the amount of time needed for a patient to rise five times from a seated position without using their arms; the timed chair-stand test is a variation that counts how many times a patient can rise and sit in the chair over a 30-second interval.
Compared with the HGS, the sit-stand test requires both strength and endurance to complete.[38]Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999 Jun;70(2):113-9.
http://www.ncbi.nlm.nih.gov/pubmed/10380242?tool=bestpractice.com
The sit-stand test was also recommended by the EWGSOP2 working group as part of the initial muscle strength assessment, particularly in settings where a hand dynamometer is not available.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
A cut-off of >15 seconds for 5 rises indicates low strength, according to the EWGSOP2.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
The AWGS uses a cut-off of ≥12 seconds for 5 rises to indicate low strength.[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
Patients identified as having low muscle strength on the HGS and/or sit-stand test are considered to have probable sarcopenia. According to the EWGSOP2 recommendations, further imaging investigations are warranted to confirm the diagnosis.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
An assessment of the patient's physical performance will help to determine the severity of the sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
Mobility, balance, and gait assessment (physical performance)
Several functional tests may be used to assess physical performance and muscle function in older adults (and therefore severity of sarcopenia) and are suitable in most clinical settings. Gait speed is recommended by EWGSOP2, ICSFR, and AWGS for evaluation of physical performance.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
Gait speed
Use gait speed as a valid measurement of muscle function.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
Gait speed is a commonly used index to evaluate mobility and muscle function. It is considered a fast, safe, and reliable examination method for evaluating gait. It involves having the patient walk a short distance (3-6 m), selecting the segment of stabilised speed within the distance, and calculating the average step speed in metres per second.[39]Miller ME, Magaziner J, Marsh AP, et al; LIFE Investigators. Gait speed and mobility disability: revisiting meaningful levels in diverse clinical populations. J Am Geriatr Soc. 2018 May;66(5):954-61.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5992037
http://www.ncbi.nlm.nih.gov/pubmed/29608795?tool=bestpractice.com
Values <1 m/second are considered low.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
Values <0.8 m/second are often used as the level to initiate screening.[40]Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547-58.
https://academic.oup.com/biomedgerontology/article/69/5/547/672497
http://www.ncbi.nlm.nih.gov/pubmed/24737557?tool=bestpractice.com
A single cut-off speed of ≤0.8 m/second indicates severe sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
The Short Physical Performance Battery (SPPB)
The SPPB combines several functional tests to measure lower extremity muscle strength by rising from a chair 5 times as quickly as possible, and dynamic balance by standing feet together in semi-tandem and tandem, and walking 4 m.
NIH National Institute on Aging: Short physical performance battery (SPPB)
Opens in new window The scores range from 0 to 12 points (optimum performance). Any value of ≤8 points is considered abnormal.[41]Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016 Oct 5;16(1):170.
https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-016-0349-4
http://www.ncbi.nlm.nih.gov/pubmed/27716195?tool=bestpractice.com
In a cross-sectional analysis of 294 community-dwelling individuals ≥65 years, the authors reported a high sensitivity of the SPPB when using the cut-point of ≤8, which suggested that it may be useful as a screening tool for sarcopenia in clinical settings where appendicular skeletal lean mass (ALM) measurements are unavailable.[42]Phu S, Kirk B, Bani Hassan E, et al. The diagnostic value of the Short Physical Performance Battery for sarcopenia. BMC Geriatr. 2020 Jul 13;20(1):242.
https://bmcgeriatr.biomedcentral.com/articles/10.1186/s12877-020-01642-4
http://www.ncbi.nlm.nih.gov/pubmed/32660438?tool=bestpractice.com
This test is more often used in research than in a clinical assessment setting because of the time it takes to administer.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
The 'timed-up-and-go' test
The timed-up-and-go test is used to assess mobility and functional capacity in older adults.[43]Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991 Feb;39(2):142-8.
http://www.ncbi.nlm.nih.gov/pubmed/1991946?tool=bestpractice.com
Participants are instructed to rise from a chair, walk 3 metres, turn around a physical marker, walk back to the chair, and sit down, while being timed. Values ≥20 seconds are a cut-off point for low performance.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
400-metre walk
A 400-metre walk is not directly a test for sarcopenia, but is another mobility assessment with an endurance component.[44]Rolland YM, Cesari M, Miller ME, et al. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004 Jun;52(6):972-6.
http://www.ncbi.nlm.nih.gov/pubmed/15161464?tool=bestpractice.com
Participants are instructed to walk at a normal pace. During the test, they are not allowed to use assistive devices, except for a cane. Time is measured using a manual chronometer. The result is considered normal if the test is completed within 6 minutes.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
Initial investigations
Request blood tests to identify any secondary causes of sarcopenia, such as electrolyte disturbances, hypogonadism, vitamin D deficiency, hyperparathyroidism, and malnutrition. Sarcopenia can be seen with impairment of several organs such as kidney disease, heart failure, and hyperthyroidism. For these reasons, the initial blood panel should include a full blood count, electrolytes, glucose, liver enzymes, kidney function, and thyroid-stimulating hormone. Depending on these results, further investigation should be pursued.
Muscle mass (muscle quantity) can be estimated in clinical practice by several imaging techniques:
Each has advantages and disadvantages related to cost, accessibility, simplicity, reliability, and duration of measurements. Recently, ultrasound has gained considerable interest as a research technique to measure muscle quantity as well as quality.
It is not always possible to have an objective measurement of muscle mass. Therefore, clinical judgement may suffice if screening tools are suggestive of sarcopenia and supported by physical examination.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
In practice, where it is not possible to measure muscle mass (or there will be delay in doing so), treatment for sarcopenia should be initiated based on low muscle strength alone.
CT and MRI
CT and MRI are recommended for estimating muscle volume and quality in patients identified clinically as having probable sarcopenia.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
Both imaging tools can measure cross-sectional areas of a specific muscle, such as the quadriceps or psoas, or provide total body muscle volumes. They can provide the amount of intramuscular fat infiltration to define muscle quality.[45]Huber FA, Del Grande F, Rizzo S, et l. MRI in the assessment of adipose tissues and muscle composition: how to use it. Quant Imaging Med Surg. 2020 Aug;10(8):1636-49.
https://qims.amegroups.org/article/view/38300/html
http://www.ncbi.nlm.nih.gov/pubmed/32742957?tool=bestpractice.com
CT delivers high radiation levels, but MRI requires the absence of metallic implants or devices such as a pacemaker.
In a study of one MRI protocol, including 468 individuals (200 women), a reduction in relative skeletal muscle mass was observed, starting in the third decade, with a noticeable decrease in absolute skeletal muscle mass starting at the end of the fifth decade. This decrease was primarily attributed to a reduction in lower body skeletal muscle.[46]Janssen I, Heymsfield SB, Wang ZM, et al. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985). 2000 Jul;89(1):81-8.
https://journals.physiology.org/doi/full/10.1152/jappl.2000.89.1.81
http://www.ncbi.nlm.nih.gov/pubmed/10904038?tool=bestpractice.com
Dual-energy x-ray absorptiometry (DXA)
DXA is the imaging technique recommended by the ICFSR to determine the level of lean body mass when diagnosing sarcopenia, due to the lower cost and less radiation exposure compared with CT and MRI.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
DXA is based on two x-ray attenuations passing through the body to accurately calculate the mass of two different tissues, namely bone and fat.[47]Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996 Jan;12(1):45-51.
http://www.ncbi.nlm.nih.gov/pubmed/8838836?tool=bestpractice.com
The difference between body weight and the sum of these two masses is used to calculate lean mass. DXA can estimate masses by body region. Only the limbs are used, in order to exclude visceral lean mass. The sum of leg and arm lean masses, which is essentially muscle mass, is called appendicular skeletal muscle (ASM). ASM divided by the person's height squared gives rise to the appendicular skeletal mass index (ASMI).
Sarcopenia was first defined by an ASMI two standard deviations below a reference population of healthy young individuals.[48]Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755-63.
https://academic.oup.com/aje/article/147/8/755/88959
http://www.ncbi.nlm.nih.gov/pubmed/9554417?tool=bestpractice.com
Using this definition, thresholds of sarcopenia were established if ASMI was <7.26 kg/m² or <5.45 kg/m² for men and women, respectively. These have remained similar in the EWGSOP2 guideline, which proposes <7.0 kg/m² and <5.5 kg/m², respectively, based on ASM <20 kg and <15 kg for men and women, respectively, and also in the AWGS, which uses <7.0 kg/m² in men and <5.4 kg/m² in women.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
[6]Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020 Mar;21(3):300-7.
http://www.ncbi.nlm.nih.gov/pubmed/32033882?tool=bestpractice.com
The Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project proposed an index of appendicular lean body mass adjusted for BMI, with cut-offs of <0.79 and <0.51 for men and women, respectively.[40]Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014 May;69(5):547-58.
https://academic.oup.com/biomedgerontology/article/69/5/547/672497
http://www.ncbi.nlm.nih.gov/pubmed/24737557?tool=bestpractice.com
In a cross-sectional study using DXA to estimate muscle mass in 433 men and women aged 18-94 years, muscle mass was almost steady from age 18 to 60 years and declined after age 60 years.[49]Kyle UG, Genton L, Hans D, et al. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001 Aug;55(8):663-72.
http://www.ncbi.nlm.nih.gov/pubmed/11477465?tool=bestpractice.com
Compared with CT and MRI as techniques for quantifying muscle mass, DXA has a reasonable reliability for quantifying lean and fat tissues, emits less radiation, and is more accessible and less expensive. However, DXA scanning is not portable, which limits its use in large epidemiological studies. Also, DXA cannot account for fatty infiltration of muscle, which can lead to an overestimation of lean mass.
Bioimpedance analysis (BIA)
BIA measures the rate at which a painless low-voltage electrical current travels through the body. It is based on the principle that different tissues in the body allow the electrical current to travel at distinct speeds. Fat is more resistant than muscle or water, so the higher the resistance, the higher the body fat percentage. Bioimpedance is a complex term composed of resistance (R) and reactance (Xc), the latter being caused by the capacitance of the cell membrane. These parameters are obtained by placing electrodes in specific body areas, such as the ankle and wrist, or by standing on a BIA scale barefoot. The frequency of the current can be either single or multiple, and body segments can be assessed.[50]Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors (Basel). 2014 Jun 19;14(6):10895-928.
https://www.mdpi.com/1424-8220/14/6/10895
http://www.ncbi.nlm.nih.gov/pubmed/24949644?tool=bestpractice.com
By entering R and Xc parameters in equations containing data on height, sex, age, and weight, body fat and fat-free masses can be calculated.[49]Kyle UG, Genton L, Hans D, et al. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr. 2001 Aug;55(8):663-72.
http://www.ncbi.nlm.nih.gov/pubmed/11477465?tool=bestpractice.com
BIA is performed in many field studies as it is inexpensive, simple to operate, and reliable. However, it is less accurate than DXA.[2]Dent E, Morley JE, Cruz-Jentoft AJ, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148-61.
http://www.ncbi.nlm.nih.gov/pubmed/30498820?tool=bestpractice.com
In addition, oedema can affect its accuracy. DXA could be considered the best method to quantify lean mass in research, whereas BIA is a valid alternative to DXA. The equipment is widely available, portable, and affordable.[1]Cruz-Jentoft AJ, Bahat G, Bauer J, et al; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019 Jan 1;48(1):16-31.
https://academic.oup.com/ageing/article/48/1/16/5126243
http://www.ncbi.nlm.nih.gov/pubmed/30312372?tool=bestpractice.com
Different machines used for BIA produce different results, which are not comparable. Also, different populations require different equations to calculate muscle mass. Using BIA in clinical practice can only be done accurately when validation of a particular machine/population combination is available. Although body composition analysis by DXA is an appropriate approach to assess sarcopenia, neither BIA nor DXA are widely used in clinical practice.
