Recommendations
Your Organisational Guidance
ebpracticenet urges you to prioritise the following organisational guidance:
Opvolging en revalidatie van patiënten met aanhoudende klachten na COVID-19 in de eerste lijnPublished by: KU Leuven | Werkgroep Ontwikkeling Richtlijnen Eerste Lijn (Worel)Last published: 2023Suivi et revalidation des patients présentant des symptômes persistants après la COVID-19 en première lignePublished by: KU Leuven | Groupe de Travail Développement de recommmandations de première ligneLast published: 2023Key Recommendations
Management predominantly depends on disease severity, and focuses on the following principles: infection prevention and control measures; symptom management; prevention of disease progression; optimised supportive care; and organ support in severe or critical illness.
Consider whether the patient can be managed at home. Generally, patients with asymptomatic or mild to moderate disease can be managed at home.[85] Provide symptom relief as necessary, including treatments for fever or cough.[85][401] Consider antiviral treatment for patients with non-severe disease who are at moderate to high risk of hospitalisation.[401][402]
Admit patients with severe disease to an appropriate healthcare facility. Assess adults for frailty on admission. Patients with critical disease require intensive care; involve the critical care team in discussions about admission to critical care when necessary. Monitor patients closely for signs of disease progression.[85][401]
Start supportive care according to the clinical presentation. This might include symptom relief, oxygen therapy, intravenous fluids, venous thromboembolism prophylaxis, high-flow nasal oxygen (HFNO), non-invasive or invasive mechanical ventilation, or extracorporeal membrane oxygenation. Sepsis and septic shock should be managed according to local protocols.[85]
Consider empirical antibiotics if there is clinical suspicion of a secondary bacterial infection. Antibiotics may be required in patients with moderate, severe, or critical disease. Give within 1 hour of initial assessment for patients with suspected sepsis or if the patient meets high-risk criteria. Base the regimen on the clinical diagnosis, local epidemiology and susceptibility data, and local treatment guidelines.[85][401]
Consider systemic corticosteroid therapy in patients with severe or critical disease. Moderate-quality evidence suggests that systemic corticosteroids probably reduce 28-day mortality in patients with severe and critical disease.[398][401][402]
Consider the antiviral remdesivir in patients with severe disease. Low-certainty evidence suggests that remdesivir possibly reduces mortality, and moderate-certainty evidence suggests that remdesivir probably reduces the need for mechanical ventilation.[398][401][402]
Consider an interleukin-6 inhibitor (tocilizumab or sarilumab) and/or a Janus kinase inhibitor (baricitinib) in patients with severe or critical disease. High-certainty evidence suggests that interleukin-6 inhibitors reduce mortality and the need for mechanical ventilation. High-certainty evidence suggests that baricitinib reduces mortality, and moderate-certainty evidence suggests that it probably reduces the duration of mechanical ventilation.[398][401][402]
Assess whether the patient requires any rehabilitation or follow-up after discharge. Discontinue transmission-based precautions (including isolation) and release patients from the care pathway according to your local guidance.[85]
Implement local infection prevention and control procedures when managing patients. For patients in home isolation, advise patients and household members to follow appropriate infection prevention and control measures:
Guidance on when to stop isolation varies widely across locations.
Isolation periods, if applicable, may depend on various factors including circulating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and patient factors (e.g., immunocompetent/immunocompromised, asymptomatic/symptomatic, disease severity).
The World Health Organization recommends 10 days of isolation for symptomatic patients, and 5 days of isolation for asymptomatic patients (based on very low-certainty evidence). Rapid antigen testing may be used to reduce the period of isolation.[85]
Some countries now recommend isolation periods as short as 5 days to 7 days, and some no longer recommend an isolation period at all.
Consult your local public health guidance for more information.
Treatment options depend on factors including disease severity, availability, and local guidelines.
The World Health Organization strongly recommends corticosteroids, interleukin-6 inhibitors, or Janus Kinase inhibitors (baricitinib) for patients with severe or critical disease, and nirmatrelvir/ritonavir for patients with non-severe disease who are at high risk of hospitalisation. It makes weak or conditional recommendations in favour of molnupiravir or remdesivir for patients with non-severe disease who are at high risk of hospitalisation, nirmatrelvir/ritonavir for patients with non-severe disease who are at moderate risk of hospitalisation, and remdesivir for those with severe disease.[402][654][655]
[Figure caption and citation for the preceding image starts]: Summary of therapeutic options from the WHOBMJ. 2023 Nov 9:383:2622 [Citation ends].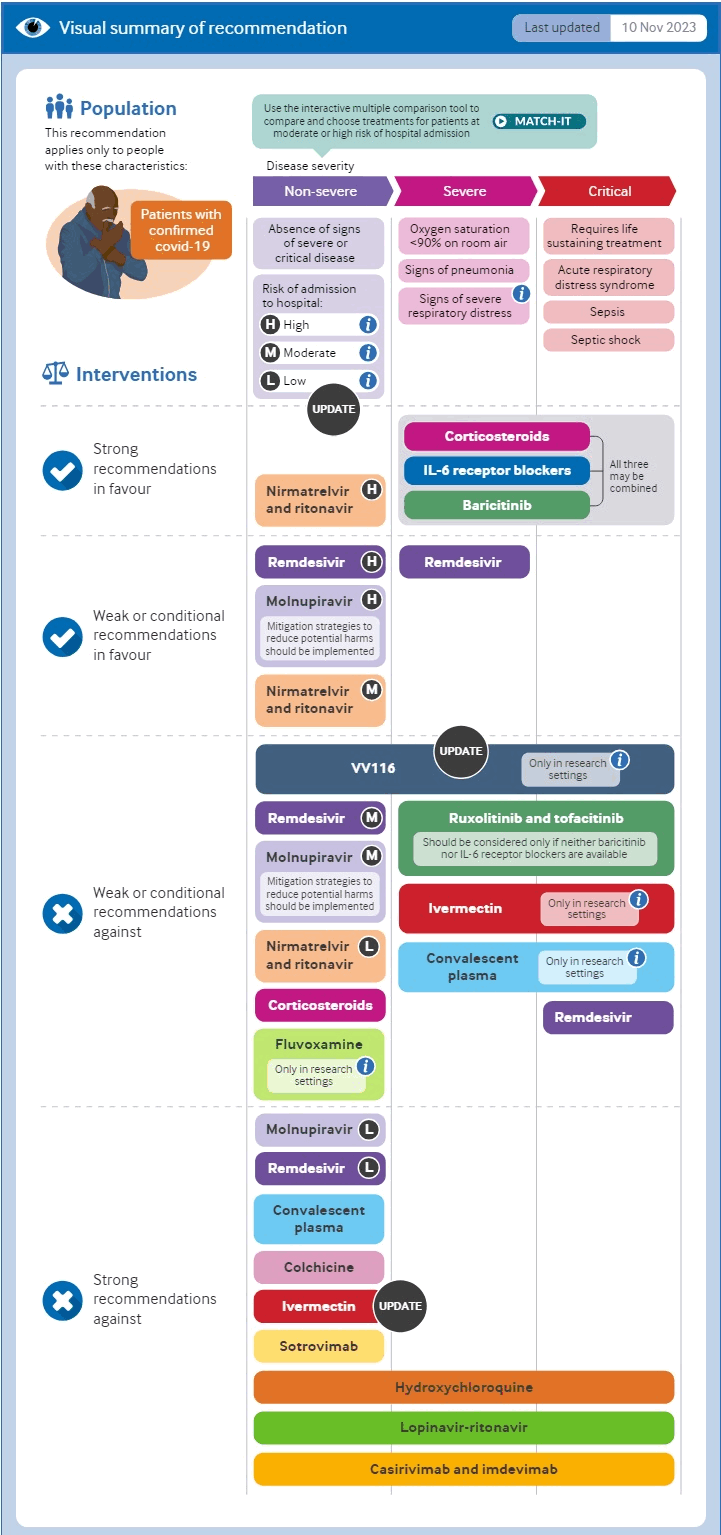
The management recommendations in this topic are based on guidelines from the World Health Organization, as well as key guidance from the UK National Institute for Health and Care Excellence and the Infectious Diseases Society of America. Consult your local guidelines for more detailed or specific information.
The majority of patients have mild to moderate illness that does not warrant medical intervention or hospitalisation, depending on the circulating SARS-CoV-2 variant. The pooled proportion of non-severe illness in people infected with the Omicron variant was 98%, and the pooled proportion of asymptomatic infection was 25% (proportions varied depending on vaccination status).[438] For disease severity definitions, see Criteria.
Location of care
Manage patients in a healthcare facility, in a community facility, or at home according to guidance from your local public health authority. Home management can be considered in most patients, with telemedicine or remote visits as appropriate. Manage patients at high risk of deterioration in a healthcare facility.[85][656]
The decision to manage patients at home requires careful clinical judgement and should be informed by an assessment of the patient’s home environment to ensure that:[656]
Infection prevention and control measures and other requirements can be met (e.g., basic hygiene, adequate ventilation)
The carer is able to provide care and recognise when the patient may be deteriorating
The carer has adequate support (e.g., food, supplies, psychological support)
The support of a trained health worker is available in the community.
Symptom management
Fever and pain: paracetamol or ibuprofen are recommended.[85][401] Ibuprofen should only be taken at the lowest effective dose for the shortest period needed to control symptoms.
Cough: advise patients to avoid lying on their back as this makes coughing ineffective, and follow your local guidelines for treating acute cough.[401]
Olfactory dysfunction: consider treatment (e.g., olfactory training, intranasal corticosteroids) if olfactory dysfunction persists beyond 2 weeks. Often it improves spontaneously and does not require specific treatment.[659][660]
A Cochrane review found there is very limited evidence regarding the efficacy and harms of different interventions for preventing or treating persistent olfactory dysfunction following infection. The only evidence available is for intranasal corticosteroids (for prevention), and this is of very low certainty, so no conclusions could be drawn.[661][662]
A systematic review and meta-analysis found that there were no significant differences in the improvement of olfactory scores with either intranasal or oral corticosteroids plus olfactory training compared with olfactory training alone. Olfactory function was significantly improved after olfactory training.[663]
Supportive care
Advise patients about adequate nutrition and appropriate rehydration. Advise patients to drink fluids regularly to avoid dehydration. Fluid intake needs can be higher than usual because of fever. However, too much fluid can worsen oxygenation.[85][401]
Advise patients to improve air circulation by opening a window or door.[401]
Provide basic mental health and psychosocial support for all patients, and manage any symptoms of insomnia, depression, or anxiety as appropriate.[85]
Antivirals
Antiviral agents are approved or have an emergency-use authorisation in most countries. Options include:
Nirmatrelvir/ritonavir: nirmatrelvir is an oral SARS-CoV-2 protease inhibitor. It is administered with a low dose of ritonavir to slow the hepatic metabolism of nirmatrelvir and increase the plasma concentration of nirmatrelvir to a therapeutic level
Molnupiravir: an oral SARS-CoV-2 nucleoside analogue
Remdesivir: an intravenous RNA polymerase inhibitor.
The World Health Organization strongly recommends nirmatrelvir/ritonavir in patients with non-severe disease who are at high risk of hospitalisation, and conditionally recommends nirmatrelvir/ritonavir in patients with non-severe disease who are at moderate risk of hospitalisation. It suggests the use of molnupiravir or remdesivir in patients with non-severe disease who are at high risk of hospitalisation if nirmatrelvir/ritonavir is not available, but suggests against the use of these agents in patients who are at moderate risk of hospitalisation. Antiviral therapy is not recommended in patients who are at low risk of hospitalisation (most patients). For risk definitions, see 'World Health Organization: hospitalisation risk for patients with non-severe disease' in Criteria.[402][654][655]
Nirmatrelvir/ritonavir is a superior choice to other treatments for non-severe disease because it may have greater efficacy in preventing hospitalisation compared with the alternatives, has fewer concerns with respect to harms than does molnupiravir, and is easier to administer than intravenous remdesivir. However, it does have significant and complex drug-drug interactions.
The recommendation to use nirmatrelvir/ritonavir is based on high-certainty evidence that suggests nirmatrelvir/ritonavir likely reduces hospital admission, and moderate- to high-certainty evidence that suggests it may reduce mortality depending on the patient’s risk of hospitalisation.
The recommendation to use remdesivir is based on moderate-certainty evidence that suggests remdesivir probably reduces hospital admission and reduces mortality and mechanical ventilation.
The recommendation to use molnupiravir is based on moderate-certainty evidence that suggests molnupiravir probably reduces hospital admission, time to symptom resolution, and mortality.
In the UK, the National Institute for Health and Care Excellence (NICE) recommends nirmatrelvir/ritonavir or molnupiravir for patients who do not need supplemental oxygen, and are thought to be at high risk of progression to severe disease.[401][664]
In addition to this, nirmatrelvir/ritonavir is also recommended in the following: people ≥70 years of age; people with a BMI ≥35 kg/m2; patients with diabetes or heart failure.
In the US, the Infectious Diseases Society of America recommends nirmatrelvir/ritonavir or remdesivir as the preferred treatment options in patients with mild to moderate disease who are at high risk for progression to severe disease.[398]
Molnupiravir is recommended only in patients who have no other treatment options (i.e., nirmatrelvir/ritonavir or remdesivir).
Treatment should be initiated as soon as possible after diagnosis, ideally within 5 days of symptom onset for nirmatrelvir/ritonavir or molnupiravir, or within 7 days of symptom onset for remdesivir.[398][401][402]
Logistical constraints may make it difficult to administer remdesivir in some outpatient settings as it requires administration via intravenous infusion.
Antivirals may not be recommended in children, pregnant women, or breastfeeding women.[398][401][402] However, recommendations vary, and you should consult your local guidelines.
Cases of virological rebound (i.e., recurrent positive polymerase chain reaction result) and the recurrence of symptoms have been reported after antiviral treatment. However, it appears to be mild and self-limited. Virological rebound was more common in patients taking nirmtarelvir/ritonavir compared with untreated patients, but was also reported in patients taking molnupiravir.[665]
Emerging data indicate that mutations associated with nirmatrelvir resistance have been identified, particularly in immunocompromised patients. However, the data suggest a low risk of significant drug resistance with current SARS-CoV-2 variants and antiviral usage patterns.[666]
Evidence for the use of antivirals in patients with non-severe disease is limited.
Nirmatrelvir/ritonavir was found to reduce the risk of hospitalisation or death by 89% (within 3 days of symptom onset) and 88% (within 5 days of symptom onset) compared with placebo in non-hospitalised, unvaccinated, high-risk adults in the phase 2/3 EPIC-HR trial.[667] However, the phase 2/3 EPIC-SR trial found that time to sustained alleviation of all signs and symptoms did not differ significantly between nirmatrelvir/ritonavir and placebo, regardless of vaccination status, in patients who were symptomatic (non-hospitalised) and at standard or high risk for severe disease.[668] Meta-analyses have found that nirmatrelvir/ritonavir reduced the risk of emergency department visits, hospitalisation, intensive care unit admission, oxygen requirement, and mortality. However, large-scale randomised controlled trials are required to confirm these findings.[669][670] One systematic review found that nirmatrelvir/ritonavir reduced the risk of mortality and hospitalisation in older patients (moderate-certainty evidence), but did not improve the outcomes of mortality and hospitalisation in patients <65 years of age (low-certainty evidence).[671] A Cochrane review found that nirmatrelvir/ritonavir may reduce the risk of all-cause mortality and hospital admission or death within 28 days in unvaccinated outpatients with previous infection who were at high risk with symptom onset within 5 days and infected with the Delta variant (low-certainty evidence from one trial). Very low-certainty evidence exists regarding the effects on all-cause mortality and viral clearance in unvaccinated inpatients with mild to moderate infection caused by the Omicron variant.[672]
Molnupiravir was found to reduce the risk of hospitalisation or death by 31% (absolute risk reduction from 9.7% to 6.8%) in the 29 days after use compared with placebo in non-hospitalised, unvaccinated, at-risk adults in the phase 3 MOVe-OUT trial.[673] However, the PANORAMIC trial found that molnupiravir did not reduce the risk of hospitalisation or death among high-risk vaccinated adults in the community compared with placebo, although it did reduce time to recovery.[674] Meta-analyses are conflicting. Some meta-analyses show no significant effect on reducing mortality or hospitalisation with molnupiravir.[675][676] However, others show that molnupiravir has a significant impact on reducing mortality and hospitalisation.[677][678] Emerging evidence suggests that use of molnupiravir may be contributing to the evolution of the SARS-CoV-2 virus, but further research is required.[679]
Remdesivir was found to reduce the risk of hospitalisation or death by 87% compared with placebo in non-hospitalised high-risk adults in a randomised, double-blind, placebo-controlled trial.[680]
When comparing nirmatrelvir/ritonavir and molnupiravir, nirmatrelvir/ritonavir demonstrated superiority to molnupiravir in terms of all-cause mortality and hospitalisation rate in one systematic review and meta-analysis. The incidence of adverse events was higher with nirmatrelvir/ritonavir, but no significant difference was observed between the two drugs in terms of adverse events that led to treatment discontinuation.[681]
Monoclonal antibodies
Monoclonal antibodies may be approved or have an emergency-use authorisation in some countries.
Monoclonal antibodies bind to non-overlapping epitopes of the receptor-binding domain of the spike protein to block virus entry into host cells.
Options may include bebtelovimab, tixagevimab/cilgavimab, casirivimab/imdevimab, sotrovimab, bamlanivimab/etesevimab, and regdanvimab, depending on your location.
Outpatient administration in specialised clinics is required as these agents are administered parenterally, which may limit their feasibility.[402]
Logistical or supply constraints may make patient triage for monoclonal antibody treatment necessary. Therapy should be prioritised for patients who are at the highest risk of progressing to severe disease.
Choice of monoclonal antibody depends on availability, as well as clinical and contextual factors including information about efficacy with different SARS-CoV-2 variants and subvariants.[401][402]
Check your local guidance for information about whether a particular monoclonal antibody is effective against current circulating SARS-CoV-2 variants and subvariants.
Treatment should be started as soon as possible and within 7 days of symptom onset.
Guideline recommendations vary.
The World Health Organization strongly recommends against the use of sotrovimab and casirivimab/imdevimab for patients with non-severe disease, as there is evidence of a reduction in effectiveness against currently circulating variants of SARS-CoV-2 and their subvariants. The agency makes no recommendations for other monoclonal antibodies, and recommends the use of antivirals instead (see above).[402][654][655]
In the UK, the National Institute for Health and Care Excellence recommends sotrovimab as an option for treating patients ≥12 years of age and ≥40 kg body weight who do not need supplemental oxygen, who have an increased risk for progression to severe disease, and for whom nirmatrelvir/ritonavir is contraindicated or unsuitable.[401][664] Tixagevimab/cilgavimab is not recommended.[682]
In the US, the Infectious Diseases Society of America does not currently recommend the use of monoclonal antibodies for the treatment of COVID-19.[398]
Evidence for the use of monoclonal antibodies in non-hospitalised patients is uncertain.
A Cochrane review found that the evidence is insufficient to draw meaningful conclusions about any specific monoclonal antibody, and the disease stage in which it should be used. Casirivimab/imdevimab decreases the risk of infection and development of clinical symptoms (high-certainty evidence). Bamlanivimab decreases the risk of infection (moderate-certainty evidence). These findings only apply to unvaccinated people, and are only applicable to variants prevailing during the study.[683]
A systematic review and meta-analysis of 27 randomised controlled trials found that monoclonal antibodies had limited effects on most of the outcomes in non-hospitalised patients with the certainty of evidence ranging from very low to moderate for most outcomes. Monoclonal antibodies reduced hospitalisation, but there were no effects on mortality.[684]
Antimicrobials
Consider empirical antibiotics in patients with moderate disease only if there is clinical suspicion of secondary bacterial infection.[85][401]
Start treatment as soon as possible, and refer to local guidelines for choice of regimen.
Do not offer an antibiotic for preventing secondary bacterial pneumonia.
Advise patients to seek medical help without delay if their symptoms do not improve, or worsen rapidly or significantly.[401]
Reconsider whether the person has signs and symptoms of more severe disease on reassessment, and whether to refer them to hospital, other acute community support services, or palliative care services.
Monitoring
Closely monitor patients (particularly those with risk factors for severe illness) for signs and symptoms of disease progression. Counsel patients about signs and symptoms of deterioration or complications that require prompt urgent care (e.g., difficulty breathing, chest pain).[85]
Pulse oximetry monitoring at home is recommended in symptomatic patients with risk factors for progression to severe disease who are not hospitalised. Patient education and appropriate follow-up are required.[85]
If the patient is being managed in hospital, monitor patients closely for signs of clinical deterioration using medical early warning scores (e.g., National Early Warning Score 2 [NEWS2]), and respond immediately with appropriate supportive care interventions.[85]
A systematic review and meta-analysis found that the NEWS2 score had moderate sensitivity and specificity in predicting the deterioration of patients with COVID-19. The score showed good discrimination in predicting the combined outcome of the need for intensive respiratory support, admission to the intensive care unit, or in-hospital mortality.[473]
Corticosteroids
Guidelines do not recommend systemic corticosteroids in patients with non-severe disease, unless there is another medical indication to do so, as they may increase the risk of mortality in these patients.[398][401][402]
Antithrombotic therapy
Guidelines published by the US Anticoagulation Forum recommend against the use of anticoagulants and antiplatelet therapy for the prevention of venous thromboembolism or arterial thrombosis in non-hospitalised patients without evidence of venous thromboembolism, unless the patient has other indications for therapy or is participating in a clinical trial.[685]
A Cochrane review found that prophylactic anticoagulation results in little or no difference in the need for hospitalisation, major bleeding, deep vein thrombosis, all-cause mortality, or adverse events compared with placebo or no treatment in non-hospitalised patients (low- to moderate-certainty evidence), but may reduce the incidence of pulmonary embolism and venous thromboembolism.[686] In people with mild disease, antiplatelet agents may result in little to no difference in 45-day mortality and serious adverse events, but may slightly reduce thrombotic events (low-certainty evidence).[687]
Highest-risk clinical groups
A UK advisory group has generated a list of conditions or cohorts who are at highest risk of serious illness from COVID-19 in the community, and who would therefore benefit from treatments (e.g., antivirals, monoclonal antibodies). This list may be used when considering the use of these treatments in adults, and includes the following: Down's syndrome and other genetic disorders; solid cancers; haematological diseases and recipients of haematological stem cell transplants; renal and liver diseases; solid organ transplant recipients; immune-mediated inflammatory disorders; respiratory diseases; immune deficiencies; HIV/AIDS; and rare neurological and severe complex life-limiting neurodisability conditions.[688] Definitions may vary across other guidelines.
Patients with suspected or confirmed severe disease are at risk of rapid clinical deterioration.[85] For disease severity definitions, see Criteria.
Location of care
Manage patients in an appropriate healthcare facility under the guidance of a specialist team.[85]
The estimated length of hospital stay is more than 10 days (mean 15 days). However, the duration depends on various factors including age, country/region, and available resources.[689]
Assessment of frailty
Use the Clinical Frailty Scale (CFS) to assess baseline health and inform discussions on treatment expectations when appropriate and within an individualised assessment of frailty. Clinical Frailty Scale Opens in new window Do not use the CFS for younger people, or for people with stable long-term disabilities (e.g., cerebral palsy), learning disabilities, or autism. Make an individualised assessment of frailty in these people, using clinical assessment and alternative scoring methods.[401]
Evidence for the use of the CFS in COVID-19 is evolving despite limitations in the reporting on its application in practice.[690]
Patients with a score between 4-9 had significantly increased mortality compared with those with a score of 1-3 in one systematic review and meta-analysis.[691] Each 1-point increase in score was associated with a 12% increase in mortality.[692] However, another systematic review and meta-analysis found that there was no difference in short-term mortality between frail and non-frail patients.[693]
A more nuanced understanding of frailty and outcomes is needed, and caution is required in placing too much emphasis on the influence of frailty on the prognosis of older people.[694]
Oxygen
Start supplemental oxygen therapy immediately in any patient with emergency signs (i.e., obstructed or absent breathing, severe respiratory distress, central cyanosis, shock, coma and/or convulsions), or any patient without emergency signs and hypoxaemia.[85]
There is no evidence of benefit for oxygen therapy in patients with COVID-19 in the absence of hypoxaemia.[695]
Target SpO₂ to ≥94% during resuscitation in adults and children with emergency signs who require emergency airway management and oxygen therapy. Once the patient is stable, a target SpO₂ >90% in children and non-pregnant adults, and ≥92% to 95% in pregnant women, is recommended. Nasal prongs or a nasal cannula are preferred in young children.[85]
Some guidelines recommend that SpO₂ should be maintained no higher than 96%.[696]
Some centres may recommend different SpO₂ targets in order to support prioritisation of oxygen flow for the most severely ill patients in hospital.
Consider positioning techniques (e.g., high supported sitting), and airway clearance management to optimise oxygenation assist with secretion clearance in adults. Consider awake prone positioning in severely ill patients who require supplemental oxygen.[85]
Awake prone positioning reduced the risk of endotracheal intubation compared with usual care. However, it did not significantly reduce mortality, ventilator-free days, length of stay in the intensive care unit or hospital, or escalation of oxygen modality. Adverse events were uncommon.[697]
Monitor patients closely for signs of progressive acute hypoxaemic respiratory failure. Patients who continue to deteriorate despite standard oxygen therapy require advanced oxygen/ventilatory support.[85]
The World Health Organization recommends HFNO, continuous positive airway pressure [CPAP], or non-invasive ventilation (helmet or face mask interface) in hospitalised patients with severe disease and acute hypoxaemic respiratory failure not needing emergent intubation, rather than standard oxygen therapy. Choice depends on factors such as availability of devices and the supply of oxygen, personal comfort and experience, and patient-specific considerations (e.g., claustrophobia with CPAP or non-invasive ventilation masks, nasal discomfort with HFNO).[85]
Symptom management and supportive care
Fluids and electrolytes: use cautious fluid management in adults and children without tissue hypoperfusion and fluid responsiveness as aggressive fluid resuscitation may worsen oxygenation.[85] Correct any electrolyte or metabolic abnormalities, such as hyperglycaemia or metabolic acidosis, according to local protocols.[698]
Fever and pain: paracetamol or ibuprofen are recommended.[85][401] Ibuprofen should only be taken at the lowest effective dose for the shortest period needed to control symptoms.
Cough: advise patients to avoid lying on their back as this makes coughing ineffective, and follow your local guidelines for treating acute cough.[401]
Breathlessness: keep the room cool, and encourage relaxation, breathing techniques, and changing body positions. Identify and treat any reversible causes of breathlessness (e.g., pulmonary oedema, pulmonary embolism, COPD, asthma).[401]
Anxiety, delirium, and agitation: identify and treat any underlying or reversible causes (e.g., offer reassurance, treat hypoxia, correct metabolic or endocrine abnormalities, address co-infections, minimise use of drugs that may cause or worsen delirium, treat substance withdrawal, maintain normal sleep cycles, treat pain or breathlessness).[85][401]
Mouth care: an important part of overall patient care in hospitalised patients who are ventilated or non-ventilated and those undergoing step-down or end-of-life care.[700]
Provide basic mental health and psychosocial support for all patients, and manage any symptoms of insomnia or depression as appropriate.[85]
Venous thromboembolism (VTE) prophylaxis
Assess bleeding risk: assess the patient’s risk of bleeding as soon as possible after admission, or by the time of the first consultant review, using a suitable risk assessment tool.[401]
If the patient is already on anticoagulation for another underlying condition, continue the current treatment dose of the anticoagulant, unless contraindicated or there is a change in clinical circumstances (e.g., bleeding develops or risk of bleeding increases).[401][685] Consider switching to low molecular weight heparin if the patient’s clinical condition is deteriorating and the patient is not currently on low molecular weight heparin.[401]
A systematic review and meta-analysis found that the use of oral anticoagulation prior to hospital admission was not associated with a reduced risk of intensive care unit admission and mortality. However, the review acknowledged that further trials are needed.[701]
Start VTE prophylaxis: start prophylaxis in all hospitalised patients, provided that there are no contraindications.[85][401][685] Start as soon as possible (within 14 hours of admission).[401]
A Cochrane review found that anticoagulants may reduce all‐cause mortality compared with no anticoagulants, but the evidence is very uncertain.[702]
A systematic review and meta-analysis found that the pooled odds of mortality between anticoagulated and non-anticoagulated hospitalised patients were similar, but lower in the standard prophylactic-dose group. Prophylactic-dose anticoagulation significantly decreased the odds of in-hospital death by 17% compared with no anticoagulation.[703]
Choice of anticoagulant: low molecular weight heparin, unfractionated heparin, or fondaparinux are the recommended options. Low molecular weight heparin is preferred over unfractionated heparin and fondaparinux, unless contraindicated.[85][401] Fondaparinux is the recommended option in patients with a history of heparin-induced thrombocytopenia.[704]
A meta-analysis found that low molecular weight heparin was associated with decreased intensive care unit admission, mechanical ventilation, hospital stay, and mortality compared with unfractionated heparin in hospitalised patients, and there was no difference in the incidence of bleeding.[705]
Mechanical thromboprophylaxis (e.g., intermittent pneumatic compression device) is recommended if anticoagulants are contraindicated or not available.[704]
There is limited evidence that direct oral anticoagulants (factor Xa inhibitors) are more effective than low molecular weight heparin in preventing VTE in hospitalised patients who are not acutely ill. However, guidelines do not recommend these agents, and further randomised controlled trials are needed.[706]
Consult a specialist for guidance on the choice and dose of anticoagulant in special patient populations (e.g., children, pregnant and breastfeeding women, hepatic or renal impairment, active cancer).
Dose of anticoagulant: standard prophylaxis doses are generally recommended over intermediate or therapeutic doses in patients without an established indication for higher-dose anticoagulation.[85][685] However, recommendations vary and you should consult your local guidance.
In the UK, the National Institute for Health and Care Excellence recommends prophylaxis doses of low molecular weight heparin. However it also makes a conditional recommendation to consider treatment doses of low molecular weight heparin in those who may benefit. The decision should be carefully considered, and choice of the most appropriate dose regimen should be guided by bleeding risk, clinical judgement, and local protocols. For those who do not need supplemental oxygen, follow standard VTE prophylaxis guidelines.[401]
A Cochrane review found that higher-dose regimens resulted in little to no difference in all-cause mortality compared with lower-dose regimens in hospitalised patients; however, higher-dose regimens were associated with an increased risk of minor bleeding up to 30 days (high-certainty evidence). Higher-dose anticoagulants probably reduce pulmonary embolism and slightly increase major bleeding compared with lower-dose regimens up to 30 days (moderate-certainty evidence). Higher-dose anticoagulants may result in little or no difference in deep vein thrombosis, stroke, major adverse limb events, myocardial infarction, atrial fibrillation, or thrombocytopenia compared with lower-dose regimens up to 30 days (low-certainty evidence).[702]
Duration of treatment: anticoagulation is generally continued until hospital discharge. Routine post-discharge VTE prophylaxis is generally not recommended, except in certain high-risk patients or if another indication for VTE prophylaxis exists.[85][685] However, in the UK, the National Institute for Health and Care Excellence recommends treatment for a minimum of 7 days, including after discharge, if standard prophylaxis doses of heparin are used.[401] If therapeutic doses of heparin are used, the recommended treatment duration is 14 days or until hospital discharge, whichever is sooner.[401] Some guidelines recommend that oral rivaroxaban (a direct oral anticoagulant) may be considered for post-discharge VTE prophylaxis.[685]
A systematic review and meta-analysis found that extended thromboprophylaxis (with either a direct oral anticoagulant or low molecular weight heparin at prophylaxis doses) administered for <35 days was significantly associated with a reduced risk of thrombosis and all-cause mortality in patients post discharge who were at high risk of thromboembolism, without increasing the risk of major bleeding.[707]
Monitoring: monitor patients for signs and symptoms suggestive of thromboembolism and proceed with appropriate diagnostic and management pathways if clinically suspected.[85] See Complications.
If the patient’s clinical condition changes, assess the risk of VTE, reassess the bleeding risk, and review VTE prophylaxis.[401]
Monitoring of clinical parameters during thromboprophylaxis depends on the anticoagulant and dose used. Consult your local protocols for more information.
Antimicrobials
Consider empirical antibiotics if there is clinical suspicion of secondary bacterial infection. Give within 1 hour of initial assessment for patients with suspected sepsis or if the patient meets high-risk criteria (or within 4 hours of establishing a diagnosis of secondary bacterial pneumonia); do not wait for microbiology results. Base the regimen on the clinical diagnosis (e.g., community-acquired pneumonia, hospital-acquired pneumonia, sepsis), local epidemiology and susceptibility data, and local treatment guidelines.[85][401]
Do not offer antibiotics for preventing or treating pneumonia if SARS-CoV-2, another virus, or a fungal infection is likely to be the cause.[401]
Consider seeking specialist advice for people who: are immunocompromised; have a history of infection with resistant organisms; have a history of repeated infective exacerbations of lung disease; are pregnant; or are receiving advanced respiratory or organ support.[401]
Seek specialist advice if there is a suspicion that the person has an infection with multidrug-resistant bacteria and may need a different antibiotic, or there is clinical or microbiological evidence of infection and the person's condition does not improve as expected after 48 to 72 hours of antibiotic treatment.
Reassess antibiotic use daily. De-escalate empirical therapy on the basis of microbiology results and clinical judgement. Regularly review the possibility of switching from intravenous to oral therapy. Duration of treatment should be as short as possible (e.g., 5 to 7 days). Antibiotic stewardship programmes should be in place.[85]
A meta-analysis found that the prevalence of antibiotic prescribing in patients with COVID-19 was 75%, which is significantly higher than the estimated prevalence of bacterial co-infection. Therefore, unnecessary antibiotic use is likely to be high in these patients.[708]
Treat laboratory-confirmed co-infections (e.g., malaria, tuberculosis, influenza) as appropriate according to local protocols.[85]
Corticosteroids
The WHO strongly recommends systemic corticosteroid therapy (low-dose intravenous or oral dexamethasone or hydrocortisone) for 7 to 10 days in adults with severe disease.[402][654][655]
This recommendation is based on moderate-quality evidence that suggests systemic corticosteroids probably reduce 28-day mortality in patients with severe disease. There is no evidence directly comparing dexamethasone and hydrocortisone. The harms of treatment in this context are considered to be minor. It is unclear whether these recommendations can be applied to children or those who are immunocompromised.
In the UK, the National Institute for Health and Care Excellence recommends offering dexamethasone (or an alternative such as hydrocortisone or prednisolone when dexamethasone cannot be used or is unavailable) for up to 10 days (unless there is a clear indication to stop early) to people who need supplemental oxygen to meet their prescribed oxygen saturation levels, or who have a level of hypoxia that needs supplemental oxygen but who are unable to have or tolerate it.[401]
In the US, the Infectious Diseases Society of America recommends dexamethasone (or an alternative corticosteroid if dexamethasone is not available) for 10 days or until hospital discharge in hospitalised patients with severe disease.[398]
Evidence supports the use of corticosteroids in hospitalised patients.
A Cochrane review found that systemic corticosteroids probably slightly reduce all-cause mortality up to 30 days in hospitalised patients with symptomatic disease (moderate-certainty evidence), although the evidence is very uncertain about the effect on all-cause mortality up to 120 days. Evidence related to the most effective type, dose, or timing of corticosteroid is uncertain.[709]
A living systematic review and network meta-analysis found that corticosteroids probably reduce mortality compared with standard care.[710][711]
Evidence suggests that higher doses may be superior to lower doses in reducing mortality in patients with severe or critical disease.[712] However, the RECOVERY trial found that higher-dose corticosteroids significantly increased the risk of death compared with usual care (which included low-dose corticosteroids) in hospitalised patients who required either no oxygen or simple oxygen only. The study continues to assess higher doses in hospitalised patients who require non-invasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).[713]
Evidence also suggests that shorter treatment courses may optimise the mortality benefit in hospitalised patients. An optimal duration of treatment was <7 days in one meta-analysis, with no additional survival benefit reported with ≥7 days of treatment.[714]
Monitor patients for adverse effects (e.g., hyperglycaemia, secondary infections, psychiatric effects, reactivation of latent infections) and assess for drug-drug interactions.
Antivirals
The World Health Organization conditionally recommends the intravenous antiviral remdesivir for 5 to 10 days in adults with severe disease. It should be initiated as soon as possible after the onset of symptoms.[402][654][655]
This recommendation is based on low-certainty evidence that suggests remdesivir possibly reduces mortality, and moderate-certainty evidence that suggests it probably reduces the need for mechanical ventilation. Moderate-certainty evidence suggests that remdesivir probably has little or no impact on time to symptom improvement. There is insufficient evidence to make a recommendation around use in children.
In the UK, the National Institute for Health and Care Excellence recommends remdesivir as an option in hospitals in adults at high risk of serious illness, and children (aged 4 weeks to 17 years and weight ≥3 kg) who have pneumonia and need supplemental oxygen or who weigh ≥40 kg and have a high risk of serious illness.[401][682]
In the US, the Infectious Diseases Society of America recommends remdesivir for 5 days in patients who require supplemental oxygen.[398]
The recommended treatment course is 5 to 10 days or until hospital discharge, whichever comes first.[398][401][402]
Evidence does not suggest any greater benefit with a 10-day course of remdesivir compared with a 5-day course, but suggests an increased risk of harm. There may be no benefit in completing the full course of remdesivir if the patient progresses.[401]
Despite guidelines recommending the use of remdesivir in patients with severe disease, evidence for its use is conflicting.
A Cochrane review found that remdesivir probably has little or no effect on all-cause mortality (up to 150 days) in hospitalised patients with moderate to severe disease compared with placebo or usual care (moderate-certainty evidence). Remdesivir probably increases the chance of clinical improvement up to day 28 slightly, and decreases the risk of clinical worsening within 28 days (moderate-certainty evidence).[715]
A 1-year follow-up of hospitalised patients in a randomised controlled trial found no long-term benefits (quality-of-life or symptom outcomes) for remdesivir compared with standard of care.[716]
Oral antivirals (nirmatrelvir/ritonavir, molnupiravir) are not currently recommended in patients with severe disease. However, there is emerging observational evidence for their use in hospitalised patients.[717]
Interleukin-6 (IL-6) inhibitors
The WHO strongly recommends a single dose of an IL-6 inhibitor (tocilizumab or sarilumab) in adults with severe disease. IL-6 inhibitors may be administered in combination with corticosteroids and Janus kinase inhibitors, and should be initiated at the same time as corticosteroids.[402][654][655]
This recommendation is based on high-certainty evidence that shows IL-6 inhibitors reduce mortality and the need for mechanical ventilation, and low-certainty evidence that suggests that IL-6 inhibitors may also reduce the duration of mechanical ventilation and hospitalisation. The evidence regarding the risk of severe adverse events is uncertain. The applicability of this recommendation to children is currently uncertain.
In the UK, the National Institute for Health and Care Excellence recommends a single dose of tocilizumab in hospitalised adults who are having systemic corticosteroids and need supplemental oxygen or mechanical ventilation.[401][664]
In the US, the Infectious Diseases Society of America recommends a single dose of tocilizumab (or sarilumab if tocilizumab is not available) in hospitalised patients with progressive severe disease who have elevated markers of systemic inflammation in addition to standard care (i.e., corticosteroids).[398]
Evidence supports the use of IL-6 inhibitors.
A Cochrane review found that tocilizumab reduced all-cause mortality at day 28 (high-certainty evidence) compared with standard care alone or placebo. The evidence suggests uncertainty around the effect of tocilizumab on mortality after day 60. Evidence for an effect on these outcomes for sarilumab is very uncertain. Tocilizumab and sarilumab probably result in little or no increase in clinical improvement at day 28 (i.e., hospital discharge or improvement measured by triallist-defined scales) (moderate-certainty evidence). The evidence for clinical improvement after day 60 is very uncertain for both drugs.[718] [
 ]
]
A living systematic review and network meta-analysis found that IL-6 inhibitors (with corticosteroids) probably reduce mortality (moderate-certainty evidence), are likely to reduce the need for mechanical ventilation (moderate-certainty evidence), and may reduce the duration of hospitalisation (moderate-certainty evidence) compared with standard care.[710][711]
IL-6 inhibitors may not be beneficial when used alone (without corticosteroids).[719]
Janus kinase (JAK) inhibitors
The WHO strongly recommends an oral JAK inhibitor (baricitinib) for 14 days or until hospital discharge (whichever is first) in adults with severe disease. Baricitinib may be administered in combination with corticosteroids and IL-6 inhibitors, and should be initiated at the same time as systemic corticosteroids.[402][654][655]
This recommendation is based on high-certainty evidence that baricitinib reduces mortality, and moderate-certainty evidence that baricitinib probably reduces the duration of mechanical ventilation and the length of hospital stay. The applicability of this recommendation to children is currently uncertain.
The WHO recommends against using other drugs within this class (tofacitinib and ruxolitinib) unless baricitinib or IL-6 inhibitors are not available as the effects of tofacitinib or ruxolitinib on mortality, need for mechanical ventilation, and hospital length of stay remain uncertain and more trial evidence is needed.
In the UK, the National Institute for Health and Care Excellence recommends baricitinib in hospitalised adults who: need supplemental oxygen, and are having or have completed a course of corticosteroids (unless contraindicated), and have no evidence of infection (other than SARS-CoV-2) that might be worsened by baricitinib. It may also be considered in children ≥2 years of age provided they meet the same criteria.[401]
In the US, the Infectious Diseases Society of America recommends baricitinib in hospitalised patients with severe disease, in addition to corticosteroid therapy. It may also be used in patients who cannot receive a corticosteroid due to a contraindication (in combination with remdesivir).[398]
Evidence supports the use of JAK inhibitors.
A Cochrane review found that JAK inhibitors probably reduced all-cause mortality up to day 28 (moderate-certainty evidence) and up to day 60 (high-certainty evidence). They probably make little or no difference in improvement in clinical status or the rate of adverse events (moderate-certainty evidence). Baricitinib was the most often evaluated JAK inhibitor.[720]
A living systematic review and network meta-analysis found that JAK inhibitors probably reduce mortality (high-certainty evidence), reduce the duration of mechanical ventilation (high-certainty evidence), and reduce length of hospital stay (high-certainty evidence) compared with standard care.[710][711]
Monoclonal antibodies
Recommendations for monoclonal antibodies in patients with severe disease differ from the recommendations for patients with mild to moderate disease. Key international guidelines do not currently recommend monoclonal antibodies for patients with severe disease.
The World Health Organization strongly recommends against the use of casirivimab/imdevimab for patients with any disease severity. The agency makes no other recommendations either for or against the use of other monoclonal antibodies in patients with severe disease.[402][654][655]
In the UK, the National Institute for Health and Care Excellence recommends not offering casirivimab/imdevimab to patients who are known or suspected to have infection caused by an Omicron variant.[401]
In the US, the Infectious Diseases Society of America does not currently recommend the use of monoclonal antibodies for the treatment of COVID-19.[398]
Evidence for the use of monoclonal antibodies in hospitalised patients is uncertain.
A systematic review and meta-analysis of 27 randomised controlled trials found that monoclonal antibodies had limited effects on most of the outcomes in hospitalised patients with the certainty of evidence ranging from very low to moderate for most outcomes. Monoclonal antibodies slightly reduced mechanical ventilation and bacteraemia, but there were no effects on mortality.[684]
Monitoring
Monitor patients closely for signs of clinical deterioration, and respond immediately with appropriate supportive care interventions.[85]
Discharge and rehabilitation
Routinely assess older patients for mobility, functional swallow, cognitive impairment, and mental health concerns, and based on that assessment determine whether the patient is ready for discharge, and whether the patient has any rehabilitation and follow-up requirements.[85]
Palliative care
Palliative care interventions should be made accessible at each institution that provides care for patients with COVID-19. Identify whether the patient has an advance care plan and respect the patient’s priorities and preferences when formulating the patient’s care plan.[85] Follow local palliative care guidelines.
There is a lack of data on palliative care in patients with COVID-19.
A rapid systematic review of pharmacological strategies used for palliative care in these patients, the first international review of its kind, found that a higher proportion of patients required continuous subcutaneous infusions for medication delivery than is typically seen in the palliative care population. Modest doses of commonly used end-of-life medications were required for symptom control. However, these findings should be interpreted with caution due to the lack of data available.[721]
Patients with critical disease (i.e., presence of acute respiratory distress syndrome, sepsis, or septic shock) should be admitted or transferred to an intensive/critical care unit. For disease severity definitions, see Criteria. The overall pooled mortality rate in patients with critical illness was 34%, and 62% of patients required mechanical ventilation. Approximately 69% of these patients had comorbidities.[722]
Use existing care bundles (i.e., three or more evidence-informed practices delivered together and consistently to improve care), chosen locally by the hospital or intensive care unit and adapted as necessary for local circumstances.[85]
Location of care
Manage patients in an intensive/critical care unit under the guidance of a specialist team.[85]
Discuss the risks, benefits, and potential outcomes of treatment options with patients and their families, and allow them to express preferences about their management. Take their wishes and expectations into account when considering the ceiling of intervention. Use decision support tools if available. Put treatment escalation plans in place, and discuss any existing advance care plans or advance decisions to refuse treatment with patients who have pre-existing advanced comorbidities.[401]
HFNO or non-invasive ventilation
The World Health Organization recommends HFNO, CPAP, or non-invasive ventilation (helmet or face mask interface) in hospitalised patients with critical disease and acute hypoxaemic respiratory failure not needing emergent intubation, rather than standard oxygen therapy.[85]
Choice depends on factors such as availability of devices and the supply of oxygen, personal comfort and experience, and patient-specific considerations (e.g., claustrophobia with CPAP or non-invasive ventilation masks, nasal discomfort with HFNO).
Consider awake prone positioning (for 8-12 hours/day, broken into shorter periods over the day) in severely ill patients who require HFNO or non-invasive ventilation.
In the UK, the National Institute for Health and Care Excellence recommends CPAP in patients with hypoxaemia that is not responding to supplemental oxygen with a fraction of inspired oxygen of ≥0.4 (40%), and escalation to invasive mechanical ventilation would be an option but it is not immediately needed or it is agreed that respiratory support should not be escalated beyond CPAP.[401]
Ensure there is access to critical care providers for advice, regular review, and prompt escalation of treatment if needed, and regular assessment and management of symptoms alongside non-invasive respiratory support.
Consider using HFNO for people when: they cannot tolerate CPAP but need humidified oxygen at high flow rates; maximal conventional oxygen is not maintaining their target oxygen saturations and they do not need immediate invasive mechanical ventilation or escalation to invasive mechanical ventilation is not suitable, and CPAP is not suitable; or they need a break from CPAP (e.g., mealtimes, skin pressure relief, mouth care), need humidified oxygen or nebulisers (or both), or need weaning from CPAP.
Do not routinely offer HFNO as the main form of respiratory support for people with respiratory failure in whom escalation to invasive mechanical ventilation would be appropriate.
Optimise pharmacological and non-pharmacological management strategies in people who need non-invasive respiratory support.
Consider awake prone positioning for hospitalised patients who are not intubated and have higher oxygen needs.
Evidence for non-invasive ventilation is limited.
There is no certain evidence that non-invasive respiratory support increases or decreases mortality in patients with COVID-19 acute respiratory failure.[725]
Limited evidence suggests that non-invasive ventilation reduces the need for intubation, improves resource utilisation, may be associated with better outcomes, and is safe.[726]
Indirect and low-certainty evidence suggests that non-invasive ventilation probably reduces mortality, similar to invasive mechanical ventilation, but may increase the risk of viral transmission. HFNO may reduce mortality compared with no HFNO.[727][728]
HFNO was superior to non-invasive ventilation for acute respiratory failure in terms of decreasing mortality. However, there was no significant difference in intubation rates and length of hospital stay between the two groups.[729][730]
HFNO may reduce intubation rate and 28-day intensive care unit mortality, and may improve 28-day ventilator-free days compared with conventional oxygen therapy in patients with acute respiratory failure. However, large-scale randomised controlled trials are necessary.[731]
The RECOVERY-RS trial (an open-label, multicenter, adaptive randomised controlled trial) found that CPAP reduced the need for invasive mechanical ventilation in adults admitted to hospital with acute respiratory failure. Neither CPAP nor HFNO reduced mortality when compared with conventional oxygen therapy.[732]
The HELMET-COVID trial (a multicentre randomised clinical trial) found that helmet non-invasive ventilation did not significantly reduce 28-day mortality compared with usual respiratory support (alternate use of mask non-invasive ventilation, HFNO, or standard oxygen according to clinical response) among patients with acute hypoxaemic respiratory failure. However, there were several important limitations to the study, and interpretation of the findings is limited by imprecision in the effect size estimate.[733]
The SOHO-COVID trial (a randomised clinical trial) found that HFNO did not significantly reduce 28-day mortality compared with standard oxygen therapy among patients with respiratory failure.[734] However, another randomised controlled trial found that treatment with HFNO reduced the likelihood of invasive mechanical ventilation and decreased the time to clinical recovery compared with conventional low-flow oxygen therapy in patients with severe disease.[735]
Airborne precautions are recommended for these interventions (including bubble CPAP) due to uncertainty about the potential for aerosolisation.[85]
However, CPAP and HFNO do not appear to be associated with significant additional air or surface viral contamination compared with supplemental oxygen.[736] Despite the trend to avoid HFNO, it has been shown to have a similar risk of aerosol generation to standard oxygen masks.[737] A systematic review and meta-analysis found no association between HFNO and non-invasive ventilation and aerosol generation-increased airborne pathogen detection.[738]
Patients with hypercapnia, haemodynamic instability, multi-organ failure, or abnormal mental status should generally not receive HFNO, although emerging data suggest that it may be safe in patients with mild to moderate and non-worsening hypercapnia. Patients with hypoxaemic respiratory failure and haemodynamic instability, multi-organ failure, or abnormal mental status should not receive these treatments in place of other options such as invasive ventilation.[85]
Monitor patients closely for acute deterioration. If patients do not improve after a short trial of these interventions, they require urgent endotracheal intubation.[85][696]
More detailed guidance on the management of acute respiratory distress syndrome (ARDS) in COVID-19 is beyond the scope of this topic; consult a specialist for further guidance.
Mechanical ventilation
Consider endotracheal intubation and invasive mechanical ventilation in patients with ARDS who are acutely deteriorating despite advanced oxygen/non-invasive ventilatory support measures.[85]
Use of mechanical ventilation in COVID-19 patients carries a high risk of mortality. Mortality is highly variable across studies, ranging between 21% and 100%. An overall in-hospital mortality risk ratio of 0.70 has been reported based on random-effect pooled estimates. Outcomes appear to have improved as the pandemic has progressed.[739] However, results have not been consistent.[740]
Early intubation may be associated with lower all-cause mortality compared with patients undergoing late intubation. However, again, results have not been consistent.[741][742]
Endotracheal intubation should be performed by an experienced provider using airborne precautions.[85]
Young children, or adults who are obese or pregnant, may desaturate quickly during intubation and therefore require pre-oxygenation with 100% FiO₂ for 5 minutes.[85]
Mechanically ventilated patients with ARDS should receive a lung-protective, low tidal volume/low inspiratory pressure ventilation strategy (lower targets are recommended in children). A higher positive end-expiratory pressure (PEEP) strategy is suggested over a lower PEEP strategy in moderate to severe ARDS. However, individualisation of PEEP, where the patient is monitored for beneficial or harmful effects and driving pressure during titration with consideration of the risks and benefits of PEEP titration, is recommended.[85][696]
Although some patients with COVID-19 pneumonia meet the criteria for ARDS, there has been some discussion about whether COVID-19 pneumonia is its own specific disease with atypical phenotypes. Anecdotal evidence from early in the pandemic suggested that the main characteristic of the atypical presentation was the dissociation between well-preserved lung mechanics and the severity of hypoxaemia.[743][744][745][746][747][748] However, this hypothesis was criticised.[749][750] A systematic review and meta-analysis published in late 2022 found no evidence for distinct respiratory system static compliance-based clinical phenotypes in patients with COVID-19-related ARDS.[751]
It has been argued that an evidence-based approach extrapolating data from ARDS not related to COVID-19 is the most reasonable approach for intensive care of COVID-19 patients.[752] However, some clinicians have warned that protocol-driven ventilator use may cause lung injury in some patients, and that ventilator settings should be based on physiological findings rather than using standard protocols. High PEEP may have a detrimental effect on patients with normal compliance.[743] Therefore, PEEP should always be carefully titrated.[753]
Consider prone ventilation in patients with severe ARDS for 12 to 16 hours per day. Pregnant women in the third trimester may benefit from being placed in the lateral decubitus position. Caution is required in children.[85][696] Longer durations may be feasible in some patients.[754]
A systematic review and meta-analysis found that there was no significant difference in mortality between prone and supine positioning, and that hospital stay was significantly higher in the prone position group. There was no difference between the two groups in terms of length of stay in the intensive care unit and days of mechanical ventilation.[755]
Lung recruitment manoeuvres are suggested, but staircase recruitment manoeuvres are not recommended.[696]
More detailed guidance on the management of ARDS in COVID-19, including sedation and the use of neuromuscular blockade during ventilation, is beyond the scope of this topic; consult a specialist for further guidance.
Inhaled pulmonary vasodilator
Consider a trial of an inhaled pulmonary vasodilator in adults and children who have severe ARDS and refractory hypoxaemia despite optimising ventilation. Taper off if there is no rapid improvement in oxygenation.[696]
A systematic review and meta-analysis found that inhaled pulmonary vasodilators may improve oxygenation, but showed no mortality benefit, compared with standard therapy.[756]
Extracorporeal membrane oxygenation
Consider extracorporeal membrane oxygenation (ECMO) according to availability and expertise if the above methods fail.[85][696]
Evidence to support the use of ECMO is limited.
A registry-based cohort study found that ECMO was associated with a 7.1% reduction in mortality in selected adults (i.e., PaO₂/FiO₂ <80 mmHg) with COVID-19-associated respiratory failure, compared with conventional mechanical ventilation without ECMO. It was most effective in patients aged <65 years and those with a PaO₂/FiO₂ <80 mmHg or with driving pressures >15 cm H₂O during the first 10 days of mechanical ventilation.[757]
Pooled mortality rates in adults receiving ECMO ranged from 39% to 49%.[758][759] The mortality rate in children was 26.6%.[760] Factors associated with an increased risk of mortality included older age, male sex, chronic lung disease, longer duration of symptoms, longer duration of invasive mechanical ventilation, higher driving pressure, and higher partial pressure of arterial carbon dioxide.[761]
There is a high risk of thrombotic and neurological complications (e.g., circuit thrombosis, major bleeding, intracranial haemorrhage, ischaemic stroke, and hypoxic ischaemic brain injury) in patients on ECMO.[762][763][764]
Management of septic shock/sepsis
The management of sepsis and septic shock in patients with COVID-19 is beyond the scope of this topic. See Complications.
Symptom management and supportive care
Consider fluid and electrolyte management, antimicrobial treatment, and symptom management as appropriate (see Severe COVID-19 above).
Venous thromboembolism prophylaxis
Recommendations for VTE prophylaxis in patients with critical disease may differ from those for severe disease (see above). Consult your local guidelines.
In the UK, the National Institute for Health and Care Excellence recommends a prophylactic dose of a low molecular weight heparin to young people and adults who need HFNO, CPAP, non-invasive ventilation, or invasive mechanical ventilation, and who do not have an increased bleeding risk. An intermediate or treatment dose of a low molecular weight heparin is only recommended in these patients as part of a clinical trial.[401]
Evidence for VTE prophylaxis is limited in patients with critical disease.
A systematic review and meta-analysis of nearly 28,000 hospitalised patients found that both intermediate-dose and therapeutic-dose anticoagulation decreased the risk of thrombotic events in critically ill patients in the intensive care unit compared with prophylactic-dose anticoagulation, but these regimens were associated with an increased bleeding risk and unchanged in-hospital mortality.[765]
Another smaller systematic review and meta-analysis found no difference between escalated doses and standard prophylaxis dosing in terms of impact on mortality in critically ill patients. However, there was a reduced risk of pulmonary embolism in the escalated dose group.[766]
Corticosteroids
The WHO strongly recommends systemic corticosteroid therapy (low-dose intravenous or oral dexamethasone or hydrocortisone) for 7 to 10 days in adults with critical disease.
This recommendation is based on moderate-quality evidence that suggests systemic corticosteroids probably reduce 28-day mortality in patients with critical disease. They also probably reduce the need for invasive ventilation.[402]
In the US, the Infectious Diseases Society of America recommends dexamethasone (or an alternative corticosteroid if dexamethasone is not available) for 10 days or until hospital discharge in critically ill patients.[398]
See the corticosteroids section under Severe COVID-19 above for more information.
Antivirals
Remdesivir may increase the risk of death in critically ill patients, and for this reason the World Health Organization, the UK’s National Institute for Health and Care Excellence, and the Infectious Diseases Society of America recommend against the routine use of remdesivir in patients with critical disease.[398][401][402]
Interleukin-6 (IL-6) inhibitors
The WHO strongly recommends an IL-6 inhibitor (tocilizumab or sarilumab) in adults with critical disease.[402][654][655]
In the UK, the National Institute for Health and Care Excellence recommends a single dose of tocilizumab in hospitalised adults who are having systemic corticosteroids and need supplemental oxygen or mechanical ventilation.[401][664]
In the US, the Infectious Diseases Society of America recommends a single dose of tocilizumab (or sarilumab if tocilizumab is not available) in critically ill patients who have elevated markers of systemic inflammation in addition to standard care (i.e., corticosteroids).[398]
See the IL-6 inhibitors section under Severe COVID-19 above for more information.
Janus kinase (JAK) inhibitors
The WHO strongly recommends an oral JAK inhibitor (baricitinib) in adults with critical disease.[402][654][655]
In the UK, the National Institute for Health and Care Excellence recommends baricitinib in hospitalised adults who: need supplemental oxygen, and are having or have completed a course of corticosteroids (unless contraindicated), and have no evidence of infection (other than SARS-CoV-2) that might be worsened by baricitinib. It may also be considered in children ≥2 years of age provided they meet the same criteria.[401]
In the US, the Infectious Diseases Society of America recommends baricitinib in critically ill patients receiving HFNO or non-invasive ventilation (and patients receiving invasive ventilation or ECMO when IL-6 inhibitors are not available), in addition to corticosteroid therapy.[398]
See the JAK inhibitors section under Severe COVID-19 above for more information.
Discharge and rehabilitation
Routinely assess intensive care patients for mobility, functional swallow, cognitive impairment, and mental health concerns, and based on that assessment determine whether the patient is ready for discharge, and whether the patient has any rehabilitation and follow-up requirements.[85]
Palliative care
Palliative care interventions should be made accessible at each institution that provides care for patients with COVID-19. Identify whether the patient has an advance care plan and respect the patient’s priorities and preferences when formulating the patient’s care plan.[85] Follow local palliative care guidelines.
There is a lack of data on palliative care in patients with COVID-19.
A Cochrane review found very low-certainty evidence for the efficacy of pharmacological interventions for palliative symptom relief, and no evidence on the safety of pharmacological interventions or safety and efficacy of non-pharmacological interventions for palliative symptom control. More evidence is needed to guide end-of-life management.[767]
A rapid systematic review of pharmacological strategies used for palliative care in these patients, the first international review of its kind, found that a higher proportion of patients required continuous subcutaneous infusions for medication delivery than is typically seen in the palliative care population. Modest doses of commonly used end-of-life medications were required for symptom control. However, these findings should be interpreted with caution due to the lack of data available.[721]
Pregnant women should be managed by a multidisciplinary team, including obstetric, perinatal, neonatal, and intensive care specialists, as well as midwifery and mental health and psychosocial support. A woman-centred, respectful, skilled approach to care is recommended.[85]
Pregnant women can generally be treated with the same supportive therapies as for non-pregnant adults, taking into account the physiological changes that occur with pregnancy.[85] However, VTE prophylaxis recommendations may differ, and the safety of antivirals in pregnancy has not been established. Despite this, it is important that pregnant women are not denied treatment inappropriately.[768]
There is significant heterogeneity in several aspects of management of pregnant women across clinical practice guidelines, especially regarding follow-up after infection and timing of delivery. However, there is a general agreement in the criteria for maternal hospitalisation and mode of delivery.[769]
Follow your local infection prevention and control procedures during labour and delivery and for newborn care. The WHO recommends that mothers and infants should remain together unless the mother is too sick to care for her baby. Breastfeeding should be encouraged while applying appropriate infection prevention and control measures (e.g., hand hygiene before and after contact with the baby, wearing a mask while breastfeeding).[85]
A detailed discussion of the management of pregnant women is beyond the scope of this topic. Consult your local protocols for more information.
Use of this content is subject to our disclaimer