Recommendations
Urgent
Assess and stabilise the patient using the Airway, Breathing, and Circulation (ABC) approach.[1][2]
Remove excess clothing and start rapid active cooling immediately based on clinical suspicion (regardless of the degree of hyperthermia or measuring technique).[1] Early cooling reduces mortality and morbidity.[1][2] Consider, if available:
Whole-body cold or iced water immersion in patients with exertional heat stroke, if practical and safe to do so[1][2]
In practice, do not use water immersion in patients with an altered level of consciousness (Glasgow Coma Scale score <15), uncooperative patients, patients who require intravenous treatment, or patients who have a history of arrhythmia (e.g., atrial fibrillation, ventricular tachycardia) or a recent cardiovascular event
Wetting and fanning the skin in patients with classic heat stroke[1]
Do not use this method in exertional heat stroke, unless water immersion or wetted ice packs are not available[1]
Wetted ice packs covering the entire body as adjunctive cooling for classic heat stroke, or for exertional heat stroke if cold water immersion is unavailable[1]
Discuss the patient with heat stroke with a senior colleague.
Monitor rectal temperature continuously.[1][2] Aim to achieve a target temperature of no less than 39.0°C.[1] Stop cooling once this temperature is reached.[1]
Give oxygen if oxygen saturation <94% and maintain at target range.[15] In patients at risk of hypercapnia prescribe oxygen if oxygen saturation <88%.[15]
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[16]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[15]
Give intravenous isotonic fluids such as normal saline (0.9% sodium chloride) or hypertonic fluids (5% dextrose in normal saline) to patients with volume depletion.[1] Use cold (4°C) intravenous fluids if available as an adjunct to active cooling.[1]
If the patient has exercise-associated hyponatraemia, give isotonic or hypertonic fluids with 3% sodium chloride.[1]
Arrange immediate transfer of the patient to the intensive care unit if they do not improve despite aggressive treatment or if they have signs of organ failure.
In the community:
Remove excess clothing and start rapid active cooling immediately (before the patient is transported to hospital) with available techniques (e.g., wetting and fanning the skin, cool water towels, wetted ice packs).[1][2] In practice, avoid using aggressive cooling techniques if you are unsure about the diagnosis
Arrange immediate transfer of the patient to hospital.[1][2]
Key Recommendations
Management of heat stroke and heat exhaustion
[Figure caption and citation for the preceding image starts]: Management of heat stroke and heat exhaustion. CHS, classic heat stroke; EHS, exertional heat strokeCreated by the BMJ Knowledge Centre [Citation ends].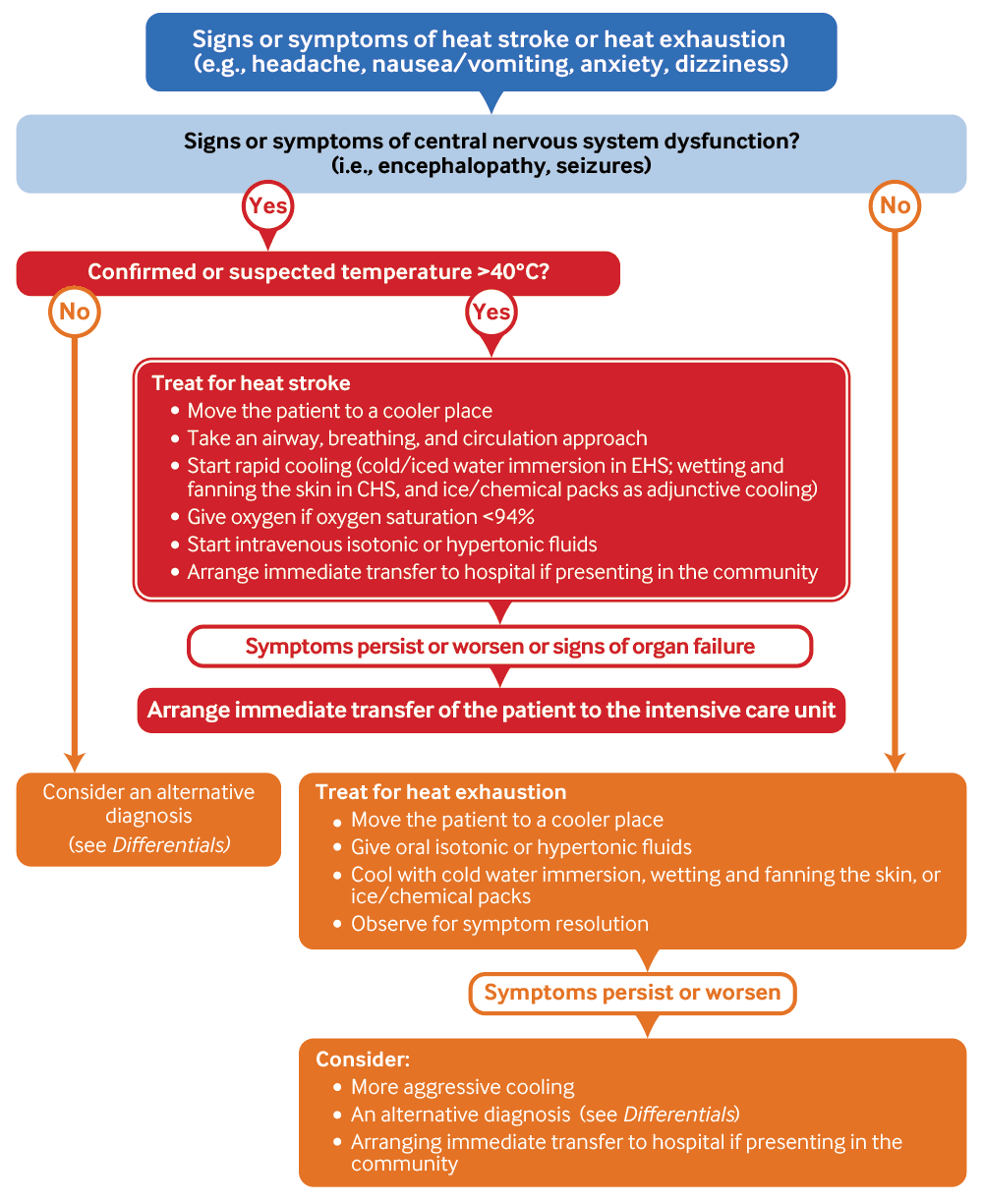
Consider giving small doses of an intravenous benzodiazepine (e.g., diazepam, midazolam) to reduce shivering, which causes heat gain (making cooling less effective).
Do not use dantrolene (usually used for treating malignant hyperthermia) in a patient with heat stroke.[1][2] Do not use antipyretics in a patient with heat stroke or heat exhaustion.[1][2]
Monitor the patient with heat stroke for complications that may develop at a later stage (even after return to normothermia) including rhabdomyolysis, acute kidney injury, disseminated intravascular coagulation, and acute liver failure.
Heat stroke can progress to multi-organ dysfunction with shock, acute respiratory failure, acute kidney injury, disseminated intravascular coagulopathy, and intestinal ischaemia.[1] The main goals of treatment are:[1]
Rapidly decreasing the patient’s core temperature to a target temperature of no less than 39.0°C
Supporting organ system function.
Remove excess clothing and start rapid active cooling immediately, based on clinical suspicion (regardless of the degree of hyperthermia or measuring techniques).[1][2]
Consider, if available:
Whole-body cold or iced water immersion (conductive cooling), if practical and safe to do so[1][2]
Guidelines recommend cold or iced water immersion as the preferred cooling method for exertional heat stroke, and the intervention can be considered in patients with classic heat stroke[1]
In practice, do not use water immersion in patients with an altered level of consciousness (Glasgow Coma Scale score <15), uncooperative patients, patients who require intravenous treatment, or patients who have a history of arrhythmia (e.g., atrial fibrillation, ventricular tachycardia), or a recent cardiovascular event
Wetting and fanning the skin (evaporative and convective cooling)[1][2]
Wetted ice packs (covering entire body) or chemical cold packs (covering cheeks, palms, and soles of the feet) as an adjunctive conductive cooling method for classic heat stroke, or for exertional heat stroke if cold water immersion is unavailable[1]
Use ice packs whenever possible as they have a greater cooling capacity than chemical cold packs[1]
Traditionally, ice packs or chemical cold packs are applied to the skin covering the neck, axillae, and groin to cool down blood passing in the major blood vessels. However, limited studies have shown no benefit in heat reduction with packs applied to these areas; ice packs are most efficacious when wet and covering the entire body and chemical cold packs are most efficacious when applied to the skin covering cheeks, palms, and soles of the feet.[1]
Aim to achieve a target temperature of no less than 39.0°C.[1] Stop cooling once this temperature is reached.[1]
Be aware that the goal of cooling is not to achieve rapid normothermia as this would result in overshoot hypothermia.[1]
Discuss the patient with heat stroke with a senior colleague.
[Figure caption and citation for the preceding image starts]: Management of heat stroke and heat exhaustion. CHS, classic heat stroke; EHS, exertional heat strokeCreated by the BMJ Knowledge Centre [Citation ends].
Evidence: Cooling methods in heat stroke
The most effective method in promoting rapid heat loss in exertional heat stroke is probably immersion in cold or iced water, although the target temperature and cooling time remain uncertain. For classic heat stroke the most effective cooling method remains controversial.
A systematic review (search date April 2006) identified four randomised controlled trials (RCTs) and 10 observational studies on cooling methods.[17]
The review included seven studies using a cooling method based on conduction (immersion in iced water), five on evaporation, and two on pharmacological cooling.
The majority of the studies were small and many were flawed. The authors discussed that it was difficult to draw firm conclusions from much of the evidence.
For exertional heat stroke, immersion in iced water was the most effective cooling method.
In young military personnel (three case series, n=41) treated to a target temperature of 38.3°C to 38.8°C, cooling time ranged from 10 to 60 minutes in all but one patient.
There were no deaths. One study reported a single patient with myocardial ischaemia (1/13, 7.7%).
Neurological morbidity (including confusion and psychosis) was rare and temporary.
One RCT (n=21) compared immersion with an evaporative cooling technique (wet towels and exposure to air 24.4°C) in hyperthermic long-distance runners. The immersion technique cooled twice as fast as the evaporative technique; however, the evaporative technique was suboptimal and morbidity and mortality were not reported.
One case series (n=36) assessed cold packs applied to the whole body (cooling time not reported). Mortality was 22.2% and neurological morbidity in survivors was 11.1%.
The evidence did not support any one cooling technique, or combination of methods, in people with classic heat stroke.
In a case series of older patients with associated comorbid illness (n=28, mean age 71 years) the cooling rate achieved with immersion was comparable to that of the younger population with exertional heat stroke.
However, mortality was 14.3% and another 14.3% sustained severe brain damage.
Immersion was also poorly tolerated and had to be converted to ice massage in some patients.
One case series (n=39) used cold packs applied to the axillae and groin and cold wet sheets applied to the trunk (in nine patients this was combined with cooling blankets or ice water lavage).
Mortality was 15% in the 27 patients with cooling time of less than 60 minutes and 33% in those with longer cooling times, although this was not statistically significant.
For evaporative techniques, in a case series (n=25) using wet gauze at 20°C cooling time ranged from 20 to 145 minutes; there was no mortality although six patients had dysfunction of ≥1 organ(s).
Another case series in patients with associated comorbid illness (n=14, mean age 66) combined water at 40°C and fan ventilation with invasive cooling methods. This enabled cooling in 34 to 89 minutes, with one death and no morbidity in survivors.
Three studies evaluated body cooling units. In two case series (n=192) the mean cooling time to reach a target temperature of 38°C was 78 minutes (range 26 to 300 minutes). The mortality rate in the studies was 11.1% and 14.9% with no neurological morbidity among survivors. A body cooling unit is a bed specially constructed to combine spraying of atomised water at 15°C and blowing of hot air at 45°C over the whole body surface to keep the wet skin temperature between 32°C and 33°C.[18]
One RCT (n=16) compared body cooling units with conventional evaporative cooling. The trial found no significant difference in cooling time, no mortality in either group, and neurological morbidity in two patients with the cooling units versus one with conventional cooling methods.
One small study (n=20) at high risk of bias found dantrolene (usually used for treating malignant hyperthermia but not recommended for heat stroke) plus evaporative cooling reduced cooling time compared with evaporative cooling alone. However, a double blind RCT (n=52) did not find any difference for cooling time, organ dysfunction, length of hospital stay, or mortality.
The target temperatures in the studies ranged from 37°C to 40.1°C. No evidence of a specific target for safe discontinuation of cooling was found.
Pharmacological treatments
Do not use dantrolene (usually used for treating malignant hyperthermia) in a patient with heat stroke.[1][2]
Dantrolene did not improve cooling rates or outcome when compared with placebo in patients with classic heat stroke.[1]
Do not use antipyretics.[1][2]
Antipyretics are not effective in reducing high body temperature related to heat. They work only when body temperature has been raised by pyrogens.[1]
Oxygen
Give oxygen if oxygen saturation <94% and maintain at target range.[15] In patients at risk of hypercapnia prescribe oxygen if oxygen saturation <88%. [15]
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia.
Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[16]
A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[15]
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[15][19]
The 2022 Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of acute asthma exacerbations.[20]
A systematic review including a meta-analysis of data from 25 randomised controlled trials published in 2018 found that, in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[16] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI 2 to 22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (relative risk 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, or patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, or sickle cell crisis).[21]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[22]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[23] The BTS guidance is due for a review in 2022.
Intravenous fluids
Give intravenous isotonic fluids such as normal saline (0.9% sodium chloride) or hypertonic fluids (5% dextrose in normal saline) to patients with volume depletion.[1] Dehydration is not a universal finding in patients with heat stroke, especially in those with classic heat stroke.[11]
In practice, you can use Hartmann's solution or Ringer’s lactate solution as they only contain small amounts of potassium and the patient with heat stroke is likely to be hyperkalaemic. If the patient has exercise-associated hyponatraemia, give isotonic or hypertonic fluids with 3% sodium chloride.[1]
Practical tip
Monitor electrolytes levels closely in patients receiving hypertonic saline.
Use cold (4°C) intravenous fluids whenever possible as an adjunct to active cooling.[1] Do not use intravascular cooling catheters or cold water lavage.[1]
Practical tip
Use intravenous fluids with caution in patients with complex medical comorbidities (e.g., heart failure, renal failure). Perform a serial clinical assessment to guide fluid therapy in these patients.
Benzodiazepines
Consider giving small doses of an intravenous benzodiazepine (e.g., diazepam, midazolam) to reduce shivering, which causes heat gain (making cooling less effective).
Small doses of benzodiazepines can make cooling more tolerable for the patient without causing sedation.
Give an intravenous benzodiazepine (e.g., lorazepam, midazolam) to control seizures.[9] Follow your local protocol.
Monitoring
Monitor temperature regularly by measuring rectal temperature or by using an oesophageal probe in intubated patients (standard practice).
Practical tip
Be cautious of falsely elevated rectal temperature measurements in the recovery phase resulting from the insulating effect of body mass.[1]
In practice, order serial bloods and monitor laboratory tests at least once daily (particularly potassium levels, renal function, arterial blood gases, clotting profile, and creatine kinase; see the Diagnosis section). If the patient is critically unwell or there is evidence of organ failure, consider repeating bloods more frequently.
Be vigilant for complications that may develop at a later stage (even after return to normothermia) including rhabdomyolysis, acute kidney injury, disseminated intravascular coagulation, and acute liver failure.[1][2] See the Complications section.
Arrange immediate transfer of the patient to the intensive care unit if they do not improve despite aggressive treatment or if they have signs of organ failure.
Consider other potential diagnoses in patients with hyperthermia and an altered level of consciousness or other non-specific symptoms, particularly if these do not resolve with rapid cooling.[1] See the Differentials section.
In the community:
Remove excess clothing and start rapid active cooling immediately (before the patient is transported to hospital) with available techniques (e.g., cold water immersion, wetting and fanning the skin, cool water towels).[1][2] In practice, avoid using aggressive cooling methods if you are unsure about the diagnosis
Arrange immediate transfer of the patient to hospital.[1][2]
If untreated, heat exhaustion can progress to heat stroke so it is important to actively reverse the process of heat exhaustion with proper cooling techniques, particularly in patients with severe heat exhaustion.[1]
See Clinical presentation in the Diagnosis section for differentiating heat exhaustion from heat stroke.
Move the patient to a cooler place and remove excess clothing.[1][2][9]
Give oral isotonic fluids (0.9% sodium chloride) or hypertonic fluids.[1]
In patients with severe heat exhaustion or nausea/vomiting, give intravenous fluids (if available).[1][9] Patients with severe heat exhaustion are more volume depleted than those with mild heat exhaustion.[1]
Patients with mild heat exhaustion generally improve with the above measures.[1] However, some patients may require active cooling, particularly those with severe heat exhaustion.[1] Consider:[1]
Cold water immersion if available and safe to use
Wetting and fanning the skin
This approach cools the patient more slowly than conductive cooling[1]
Applying wetted ice packs to cover the entire body or chemical cold packs to cover the cheeks, palms, and soles of the feet
Traditionally, ice packs or chemical cold packs are applied to the skin covering the neck, axillae, and groin to cool down blood passing in the major blood vessels. However, limited studies have shown no benefit in heat reduction with packs applied to these areas; ice packs are most efficacious when wet and covering the entire body and chemical cold packs are most efficacious when applied to the skin covering cheeks, palms, and soles of the feet.[1]
Do not use antipyretics.[1][2]
Antipyretics are not effective in reducing high body temperature related to heat. They work only when body temperature has been raised by pyrogens.[1]
Observe the patient for symptom resolution.[1][2] Symptoms typically resolve within 2 to 3 hours.[2]
If symptoms persist or worsen, consider:[1]
More aggressive cooling
An alternative diagnosis (see the Differentials section)
Arranging immediate transfer of the patient to hospital if presenting in the community.
Use of this content is subject to our disclaimer