Recommendations
Urgent
Think 'Could this be sepsis?' based on acute deterioration in a patient in whom there is clinical evidence or strong suspicion of infection.[16][17][18]
Use a systematic approach, alongside your clinical judgement, for assessment; urgently consult a senior clinical decision-maker (e.g., ST4 level doctor in the UK) if you suspect sepsis.[16][17][18][19]
Refer to local guidelines for the recommended approach at your institution for assessment and management of the patient with suspected sepsis.
Regard a hot, swollen, acutely painful joint with restriction of movement as septic arthritis until proven otherwise.[10] Do so even in the absence of fever and irrespective of microbiology and blood test results.[10]
Delayed diagnosis and treatment can result in permanent joint damage with resulting disability.[20] The mortality rate is 11% to 50%.[4][21]
Any joint can be affected; large joints (e.g., knee and hip) are most commonly reported.[10]
Prosthetic joint (to orthopaedics)
Inaccessible joint, such as hip (to emergency physician or orthopaedics and imaging).
Otherwise, take a synovial fluid sample, bloods, and any other relevant culture samples before starting antibiotics, unless this would cause undue delay.[10]
Aspirate the joint through a closed-needle approach using sterile technique if it is safe and you have had appropriate training and experience of this procedure.
Assess the colour, viscosity, and clarity of the joint aspirate to support/weaken your presumptive diagnosis.
Aspirate to dryness.[10] Repeat aspiration to dryness as often as is required, for:
Pain relief
Removing source of sepsis
Diagnosis/monitoring.
Send joint aspirate to the microbiology laboratory for urgent processing.[10] In practice, it is important that you chase the results. Order:
Gram stain, microscopy, and white cell count
Polarising microscopy for crystals
Culture and sensitivities.
As in any suspected bacterial infection:
Send blood samples for:[10]
Culture and sensitivities
White cell count
Erythrocyte sedimentation rate and C-reactive protein
Urea, electrolytes, and liver function tests.
Take swabs:
For MRSA in patients who have recently been in hospital or a nursing home, as joint aspirate does not always yield cultures and MRSA carriage will affect management[10]
From any other sources of potential infection identified on history and examination (e.g., pressure sores, skin lesions, genitourinary tract, respiratory tract).[10]
Key Recommendations
[Figure caption and citation for the preceding image starts]: Flowchart for diagnosis and management of septic arthritisCreated by the BMJ Knowledge Centre [Citation ends].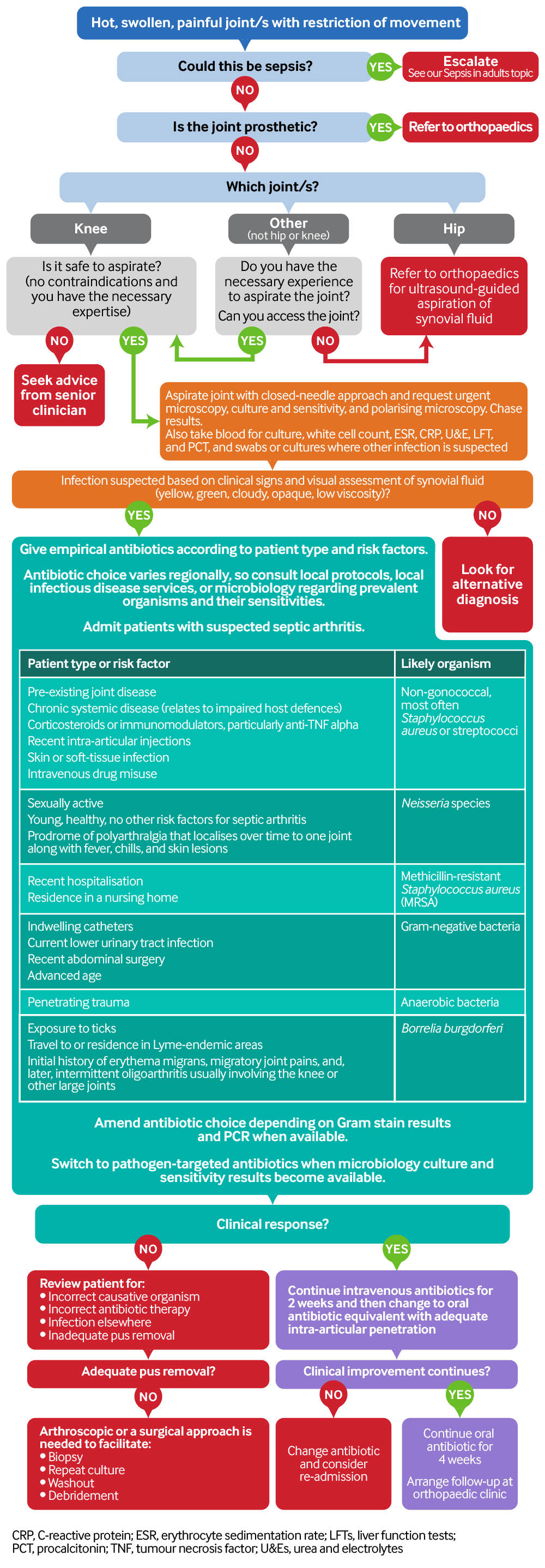
Presentation
Pain and swelling are the most common symptoms.[10]
In patients with underlying joint disease, suspect a septic joint if symptoms are out of proportion to disease activity elsewhere.[10]
Do not exclude the diagnosis of septic arthritis in patients with polyarticular disease. Up to 22% of patients with septic arthritis have oligoarticular or polyarticular disease.[10]
A hot, swollen, painful metatarsophalangeal joint of the great toe is the commonest joint to present in primary care, is almost always due to gout, and does not require joint aspiration for diagnosis.[10]
Patients typically present with an acute (<2 weeks) history.[10]
Presentation may be more insidious in the context of a low-virulence organism or tuberculosis, or if the joint is prosthetic.[4][5]
History
Ask about factors that might indicate the infecting organism:
Non-gonococcal[10]
Joint disease
Chronic systemic disease (relates to impaired host defences)
Corticosteroids or immunomodulators, particularly anti-tumour necrosis factor alpha
Recent intra-articular injections
Skin or soft-tissue infection
Intravenous drug misuse
Gonococcal[10]
Sexually active, young, healthy, no other risk factors for septic arthritis
Either localised septic arthritis, or an arthritis-dermatitis syndrome characterised by malaise, polyarthralgias tenosynovitis, and dermatitis.[9]
MRSA[10]
Recent hospitalisation or residence in a nursing home
Gram-negative[10]
Indwelling catheters or current lower urinary tract infection
Recent abdominal surgery
Advanced age
Anaerobic
Exposure to ticks
Lyme-endemic areas.
Examination
Examine the affected joint/s carefully.
Note the position of the affected joint.
Septic joints will be held in a position of maximum joint volume: fully extended knee; hip abducted, flexed, and externally rotated.
Ensure you positively identify a joint effusion (not just surrounding soft-tissue swelling).
Note any localised swelling external to the joint, as this indicates a bursitis or cellulitis rather than a septic arthritis.
Assess the patient’s pain.
Passive and active movement of the joint will be limited and very painful in septic arthritis.
In practice, most patients with septic arthritis of a weight-bearing joint will not be able to walk.
Obtain results of synovial fluid microscopy. Note that:
White cell count is the first result available and is the most useful in differentiating between septic arthritis and other diagnoses. Non-gonococcal septic arthritis will typically have white cell counts greater than 100,000/ per mL and >75% neutrophils compared with other differentials, which will have white cell counts of 2000 to 50,000 and neutrophils <50%
In practice, however, synovial fluid white cell count is not specific enough to accurately differentiate between septic and aseptic inflammation and must be assessed in the clinical context
Crystal arthropathy can be comorbid with septic arthritis, so the presence of crystals does not preclude septic arthritis if clinical signs and symptoms suggest the diagnosis.[10]
Other investigations
Arrange other investigations.
Regard a hot, swollen, acutely painful joint with restriction of movement as septic arthritis until proven otherwise, even in the absence of fever, and irrespective of microbiology and blood test results.[10]
Fever is present in approximately 60% of cases.[10]
Septic arthritis in a single joint has a case fatality of 11%.[10] Up to 50% mortality has been quoted in cases of polyarticular sepsis.[21][23][24][25][27] Delayed diagnosis and treatment, or inadequate treatment, may lead to permanent joint damage with resulting disability.[10][20]
Onset of joint pain is typically 2 weeks or less.[10]
A more insidious presentation is associated with:[4][5]
Low-virulence organisms
Tuberculosis
Prosthetic joint.
Any joint/s can be affected.
The commonest reported site of isolated septic arthritis is the knee.[9][10] The hip, shoulder, ankle, elbow, and wrist are also common sites of joint infection.[9]
The metatarsophalangeal joint of the great toe is the commonest joint to present as hot, swollen, and painful in primary care, but this presentation is unlikely to be caused by septic arthritis. This is almost always due to gout and can be diagnosed clinically without needle aspiration. Inadequate recovery requires referral.[10] See Gout.
Infection of axial joints, such as the sternoclavicular or sacroiliac joint, is more common in patients with a history of intravenous drug misuse.[9]
Do not exclude the diagnosis of septic arthritis in patients with polyarticular signs and symptoms. Up to 22% of patients with septic arthritis have oligoarticular or polyarticular disease.[10]
In patients with underlying joint disease (such as rheumatoid arthritis or osteoarthritis), suspect a septic joint if symptoms are out of proportion to disease activity elsewhere.[10]
Fever, chills, and rigors may be present in some patients.[9][28]
Sensitivities for septic arthritis of constitutional symptoms are:[28]
Fever 57%
Chills 27%
Rigors 19%.
Practical tip
Think 'Could this be sepsis?' based on acute deterioration in a patient in whom there is clinical evidence or strong suspicion of infection.[16][17][18] See Sepsis in adults.
The patient may present with non-specific or non-localised symptoms (e.g., acutely unwell with a normal temperature) or there may be severe signs with evidence of multi-organ dysfunction and shock.[16][17][18]
Remember that sepsis represents the severe, life-threatening end of infection.[29]
Use a systematic approach (e.g., national early warning score [NEWS] 2), alongside your clinical judgement, to assess the risk of deterioration due to sepsis.[16][17][30][31] Consult local guidelines for the recommended approach at your institution.
Arrange urgent review by a senior clinical decision-maker (e.g., ST4 level doctor in the UK) if you suspect sepsis:[19]
Within 30 minutes for a patient who is critically ill (e.g., NEWS2 score of 7 or more, evidence of septic shock, or other significant clinical concerns).
Within 1 hour for a patient who is severely ill (e.g., NEWS2 score of 5 or 6).
Follow your local protocol for investigation and treatment of all patients with suspected sepsis, or those at risk. Start treatment promptly. Determine urgency of treatment according to likelihood of infection and severity of illness, or according to your local protocol.[19][31]
Always ask whether the patient has a prosthetic joint.
Refer patients with prosthetic joints to the orthopaedic team for management.[22]
Do not aspirate a prosthetic joint.[10]
Enquire about the length of the symptom history.
Symptoms have usually been present for <2 weeks at presentation.[4][5][10]
Delays in presentation may occur with low-virulence organisms, tuberculosis, or prosthetic infections.[4][5][10]
Screen for the following risk factors:[9]
Underlying joint disease
Immunosuppression
Contiguous spread
Haematogenous spread
Iatrogenic
Exposure to ticks may indicate arthritis associated with Lyme disease. Note travel to or residence in:
Many parts of the US: Eastern states (mainly New England and the mid-Atlantic), Northern midwestern states (especially Wisconsin, Minnesota, and the Great Lakes region), and the West Coast (particularly northern California and, less commonly, Oregon and Washington)[32]
Grassy/wooded areas in the UK and the Highlands of Scotland[14]
Central Europe, especially Austria, Czech Republic, southern Germany, Switzerland, Slovakia, and Slovenia[33]
Some parts of Asia (Lyme disease is well established in China).[34]
Ask about factors that might indicate the infecting organism:
Non-gonococcal (91% are streptococcal or staphylococcal):[10]
Joint disease (septic arthritis is more common in pre-existing joint disease)
Chronic systemic disease (relates to impaired host defences)
Corticosteroids or immunomodulators, particularly anti-tumour necrosis factor alpha
Recent intra-articular injections
Skin or soft-tissue infection
Intravenous drug misuse
Gonococcal:[10]
Sexually active, young, healthy, no other risk factors for septic arthritis
Either localised septic arthritis, or an arthritis-dermatitis syndrome characterised by malaise, polyarthralgias tenosynovitis, and dermatitis.[9]
MRSA:[10]
Recent hospitalisation or residence in a nursing home
Gram-negative:[10]
Indwelling catheters or current lower urinary trac t infection
Advanced age
Anaerobic:
Borrelia burgdorferi:
History of residence in or travel to Lyme-endemic regions; high-risk areas include:
Grassy/wooded areas in southern England and the Scottish Highlands[14]
Many parts of the US: Eastern states (mainly New England and the mid-Atlantic), Northern midwestern states (especially Wisconsin, Minnesota, and the Great Lakes region), and the West Coast (particularly northern California and, less commonly, Oregon and Washington)[32]
Central Europe, especially Austria, Czech Republic, southern Germany, Switzerland, Slovakia, and Slovenia[33]
Some parts of Asia (Lyme disease is well established in China)[34]
Initial history of erythema migrans, migratory joint pains, and, later, intermittent oligoarthritis usually involving the knee or other large joints[14]
See the UK National Institute for Health and Care Excellence guideline on Lyme disease for further details. NICE: Lyme disease Opens in new window
Cardinal signs of a septic joint include:[10]
Pain
Redness
Swelling
Heat
Restriction of movement.
Pain and swelling are the most common symptoms in septic arthritis.[10]
Examine the joint/s carefully with particular attention to the following:
Look for redness and any obvious swelling
Note the position of the affected joint
Septic joints will be held in a position of maximum joint volume: fully extended knee; hip abducted, flexed, and externally rotated[10]
Feel for any heat
Assess pain and movement
If the affected joint is not weight-bearing, the patient with septic arthritis will be extremely reluctant to let you examine it
Intra-articular pathology is indicated by severe limitation of active and passive movement
Practical tip
Most patients with septic arthritis of a weight-bearing joint will not be able to walk.
Synovial fluid
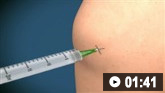
If you suspect septic arthritis, always aspirate a native joint using aseptic technique and a closed-needle approach before giving antibiotics if it is safe and you have had appropriate training and experience of this procedure.
Although this approach is appropriate in practice, it is worth bearing in mind that the most recent UK guidelines stipulate that ‘GPs and ED doctors should refer patients with suspected septic arthritis to a specialist within the hospital who has the expertise to aspirate the joint’.[10]
Always refer prosthetic joints to orthopaedics. Only a specialist should manage suspected septic arthritis in a prosthetic joint, as the diagnostic approach and management is significantly different to native joint infection, and may or may not require surgery.[10][22] Arthrocentesis should be performed in a sterile operating theatre environment.[10]
Non-specialist doctors will typically be able to aspirate the knee joint; consider seeking assistance from a senior colleague if aspirating any joint other than the knee.
If the hip is involved, refer to orthopaedics immediately for ultrasound-guided joint aspiration and possible surgical debridement.[10]
There is a lack of detail on specific contraindications for synovial fluid aspiration in native joints, and differences exist between guidelines.[10][36][37] Absolute and relative contraindications are debated, so seek advice from a senior clinician in the following circumstances:
Overlying skin infection or broken skin (as recommended by the Italian Society of Rheumatology)[36]
Coagulopathy
Treatment for anticoagulation (although the British Society of Rheumatology guidelines state that anticoagulation with warfarin is not a contraindication to needle aspiration, based on expert opinion).[10]
Do not delay giving antibiotics, however, if a senior clinical decision-maker makes a diagnosis of suspected sepsis in an acutely unwell patient with likely infection.[16][17]
Aspirate to dryness.[10] Repeat aspiration to dryness as often as is required. Aspiration has three benefits:
Pain relief
Therapeutic in removing source of infection
Diagnostic/monitoring.
Practical tip
In practice, you can use as many syringes as necessary to aspirate the joint to dryness. Simply detach the first syringe from the needle and attach the next using aseptic technique. Note that the larger the syringe (e.g., 20 mL) the larger the force required to aspirate, especially if the synovial fluid is very thick.
How to aspirate synovial fluid from the knee and administer intra-articular medication using a medial approach.
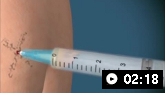
How to aspirate synovial fluid from the shoulder and administer intra-articular medication. Video demonstrates a posterior approach to the glenohumeral joint and a lateral approach to the subacromial space.
Assess the colour, viscosity, and clarity of the joint aspirate (see table below) to support/weaken your presumptive diagnosis.
Characteristics of synovial fluid in differential diagnosis[9][44]
Diagnosis | Colour | Lucency | Viscosity | Other features |
|---|---|---|---|---|
Normal | Clear | Transparent | Thick | Very few WBCs |
Non-inflammatory (e.g., osteoarthritis) | Straw | Transparent | Thick | Very few WBCs (up to 2000) Gram stain negative |
Inflammatory: crystalline disease | Yellow | Cloudy | Thin | WBC count variable Crystals present Gram stain negative |
Inflammatory: non-crystalline disease | Yellow | Cloudy | Thin | WBC count variable Gram stain negative No crystals |
Infectious: Lyme disease | Yellow | Cloudy | Thin | WBC count variable No crystals Gram stain negative Polymerase chain reaction (PCR) usually positive |
Infectious: gonococcal | Yellow | Cloudy-opaque | Thin | High WBC count (>30,000) Gram stain negative or positive No crystals PCR usually positive |
Infectious: non-gonococcal | Yellow-green | Opaque | Thin | Very high WBC count (>50,000) Gram stain usually positive No crystals |
Send joint aspirate to the microbiology laboratory for urgent processing.[10] In practice, it is important that you chase the results.
Microscopy, white cell count and Gram stain[10]
The synovial fluid white cell count is neither 100% sensitive nor 100% specific, although a count of >100,000 cells/microlitre significantly increases the likelihood of the diagnosis of infection.[28]
Synovial fluid white cell count is the first result available and is the most useful in differentiating between septic arthritis and other diagnoses. Non-gonococcal septic arthritis will typically have white cell counts greater than 100,000 per mL and >75% neutrophils compared with other differentials, which will have white cell counts of 2000 to 50,000 and neutrophils <50%.
In practice, however, synovial fluid white cell count is not specific enough to definitively differentiate between septic and aseptic inflammation and must be assessed in the clinical context.
A white blood cell count of >50,000 per mm³ and a polymorphonuclear cell count >90% have been correlated with septic arthritis, but also with crystal arthritis, which can co-exist with septic arthritis.[9][24][28]
Culture and sensitivities[10]
Microscopic analysis and culture may reveal the causative organism and its sensitivities.[10]
Synovial fluid culture is positive in:[9]
More than 90% of non-gonococcal arthritis
25% to 70% of patients with gonococcal arthritis
80% of cases of tuberculosis (although this is rare in the UK).
A negative result does not exclude the diagnosis of septic arthritis.[10]
Borrelia burgdorferi cannot be cultured from synovial fluid.
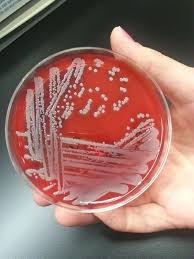
[Figure caption and citation for the preceding image starts]: Culture of Staphylococcus aureus on a blood agar plate. This is the most common cause of septic arthritisValugi / CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0) [Citation ends].
Polarising microscopy for crystals[10]
Polymerase chain reaction (PCR) for Neisseria gonorrhoeae or Borrelia burgdorferi (Lyme disease) is not routine.[10][45][46]
Practical tip
Microscopy and Gram stain are not 100% sensitive. Diagnose septic arthritis based on clinical suspicion.[10]
Blood
Draw blood for culture and sensitivities before giving antibiotics (for targeted antibiotic therapy).[10]
Because of haematogenous spread of infection, blood cultures are positive in at least one third of patients with septic arthritis.[10][49]
In some cases the blood culture may be positive in the absence of a positive synovial culture.
A negative result does not exclude the diagnosis of septic arthritis.[10]
Also take blood samples for the following tests:
White blood cell (WBC) count[10]
Can help inform a diagnosis, but clinical judgement and results of synovial fluid microscopy are more important.[10]
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)[10]
Can help inform a diagnosis, but clinical judgement and results of synovial fluid microscopy are more important.[10]
Urea and electrolytes
These provide baseline parameters and help assess for sepsis and end-organ damage as these may influence antibiotic choice.[10]
Serum urate does not assist with differentiating between gout and infection.[10]
Liver function tests
These provide baseline parameters and help assess for sepsis and end-organ damage as these may influence antibiotic choice.[10]
Serum procalcitonin (PCT)
Serum PCT is a peptide precursor of the hormone calcitonin. In healthy individuals levels remain low (<0.1 ng/mL), but they rise very sharply in the presence of bacterial endotoxin.
Studies in systemic and respiratory infection have suggested that it can discriminate between bacterial and non-bacterial sources of inflammation (e.g., rheumatoid arthritis).[26]
Small studies have investigated the use of serum PCT in diagnosis of musculoskeletal infection and management.[50][51] Serum PCT may be raised in septic arthritis; a cut-off level of greater than 0.5 ng/mL might be a more specific marker for bacterial infection than CRP, ESR, or WBC count.[51][52]
Serum PCT cannot yet be recommended as a routine diagnostic tool.
Serial measurements may indicate response to therapy.[26][53]
Evidence: Procalcitonin in identifying bacterial infection
There is evidence that procalcitonin levels may support a diagnosis of septic arthritis but should not overrule clinical suspicion.[53]
The British Society for Rheumatology (BSR) 'Hot Joint update' published in March 2017 highlights procalcitonin as a promising diagnostic test but one that cannot yet be recommended routinely.
The BSR states that procalcitonin has been suggested as a biomarker for bacterial joint infection because it is usually at a low level in healthy people (<0.1 ng/mL) but rises with bacterial endotoxins.
However, evidence at the time of the update was inconclusive, and the BSR emphasises that clinical suspicion remains the mainstay of diagnosis.
A systematic review published in August 2017 included 10 studies involving a total of 838 patients and reported an overall sensitivity of serum procalcitonin levels for the diagnosis of septic arthritis of 0.54 (95% CI 0.41 to 0.66) and a specificity of 0.95 (95% CI 0.87 to 0.98).[52] Nine out of 10 studies used procalcitonin cut-off levels of 0.5 ng/mL.
In a more recent study involving 98 patients (18 in the 'gout' group, 26 in the 'calcium pyrophosphate deposition arthritis' group, 16 in the 'mechanical' [osteoarthritis or post-traumatic arthritis] group, 18 in the 'chronic inflammatory rheumatic' group, and 20 in the 'sepsis' group), at a cut-off of 0.5 ng/mL procalcitonin sensitivity was 65% and specificity was 91%. However, the serum procalcitonin levels did not differ between patients with septic or gouty arthritis.[54]
Imaging
Arrange an x-ray.[10]
Not urgent, as is not diagnostic for septic arthritis.
Provides baseline for assessing future joint damage.
Chondrocalcinosis suggests pyrophosphate arthropathy.
If hip sepsis is suspected, aspiration should be performed by the orthopaedic team under ultrasound guidance.[9][10]
Swabs and cultures
Take swabs and cultures from any other sources of potential infection identified on history and examination before giving antibiotics, for example:[10]
Pressure sores
Skin lesions
Genitourinary tract (e.g., chronic urinary tract infection, older patients, cervix, urethra, and rectum in sexually active patients)
Respiratory tract (e.g., pharynx in suspected gonococcal arthritis, sore throat).
Obtain a urine sample for urine microscopy, culture, and sensitivity in patients with indwelling catheters or recurrent urinary tract infections, as these may be a source for haematogenous spread of infection.
Practical tip
Patients with an indwelling urinary catheter often have a positive dipstick without actual infection. Await culture confirmation before assuming a urinary tract infection is the cause of immobility and confusion in an older person. Be careful not to miss septic arthritis.
Imaging
Magnetic resonance imaging is not routine. Only arrange if you suspect osteomyelitis.[10]
Other
If you suspect Lyme arthritis, request an enzyme-linked immunosorbent assay (ELISA) for Borrelia burgdorferi, followed by Western blot if the ELISA is equivocal or positive. See the UK National Institute for Health and Care Excellence guideline on Lyme disease for detailed information on testing. NICE: Lyme disease Opens in new window
Synovial biopsy for Mycobacterium tuberculosis is positive in about 95% of patients with tuberculosis and can also identify fungal infections.[55][56]
Discriminating between septic and aseptic joint inflammation is still a challenge. No test has yet been shown to be clinically useful enough to enter into routine practice. One study on synovial fluid calprotectin levels found that septic arthritis could be discriminated from non-septic inflammatory arthritides, with 76% sensitivity, 94% specificity, and a positive likelihood ratio of 12.2 at the threshold for calprotectin of 150 mg/L.[57] Further research is required to determine its clinical utility.
Use of this content is subject to our disclaimer