Recommendations
Urgent
Subarachnoid haemorrhage (SAH) is an acute life-threatening condition.[37] If interventional treatment is planned, this should be carried out at the earliest opportunity to prevent rebleeding. The risk of rebleeding is highest within 24 hours of the onset of symptoms.[37]
As soon as the diagnosis of SAH is confirmed, urgently discuss with a specialist neurosurgical neurosciences centre the need for transfer of care of the patient to the specialist centre.[37]
Assess the patient's level of consciousness (use the Glasgow Coma Scale [GCS]).[39]
In patients with GCS score ≤8 or falling, use an ABC approach.[39]
Protect the airway with simple airway manoeuvres and adjuncts (e.g., oropharyngeal or nasopharyngeal airways).
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[45] A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[46]
Call the anaesthetist. These patients require intubation, deep sedation, and sometimes paralysis.
Cerebroprotective induction is required to protect against laryngoscopy-induced increases in intracranial pressure.
Use isotonic saline as fluid resuscitation to restore normovolaemia.[39]
Start with 3 L/day (isotonic/normal saline 0.9%), and adjust infusion for oral intake and supplement other electrolytes as necessary.[39]
Avoid all hypotonic fluids.
Check pupils for size, shape, and reactivity to light every 20 minutes once patient is sedated and paralysed. Discuss any pupil changes with a neurosurgeon.
Monitor for arrhythmias that can cause haemodynamic instability.
Once you have confirmed the diagnosis of SAH:
Consider enteral nimodipine.[37]
In practice, the decision to start nimodipine should be made by a neurosurgeon; if a neurosurgeon is not available, a critical care specialist should make this decision.
An interventional neuroradiologist and a neurosurgeon should decide the best mode of intervention to manage the culprit aneurysm, taking into account the patient’s clinical condition, the characteristics of the aneurysm, and the amount and location of subarachnoid blood.[37][39] A proposed treatment plan should be documented, in discussion with the patient, and their family or carers if appropriate, based on the following options:[37]
Interventional treatment with endovascular coiling or neurosurgical clipping. If interventional treatment is planned, this should be carried out at the earliest opportunity to prevent rebleeding. The risk of rebleeding is highest within 24 hours of the onset of symptoms.[37]
No interventional procedure, with monitoring to check for clinical improvement and reassess the options for treatment.
Observe continuously for signs of deterioration.[40]
If the patient is acutely deteriorating (e.g., new focal neurological deficit, seizure, or sudden drop in the patient's level of consciousness) have a high suspicion for rebleed or acute hydrocephalus.
Consult with a neurosurgeon and arrange an urgent non-contrast CT head rescan.
Consult immediately with a neurologist or a neurosurgeon if the patient has clinically apparent seizures.
If the patient has cardiac complications (e.g., arrhythmias), seek specialist advice.
Key Recommendations
Monitoring
Patients with SAH are at risk of haemodynamic instability and neurological deterioration.
Monitor (at least until occlusion of aneurysm):[39]
Neurological status and examination at least every hour
Use the Glasgow Coma Scale (serial GCS recordings) and perform a gross neurological examination [ Glasgow Coma Scale Opens in new window ]
Consult with a neurosurgeon if there are any pupil changes or signs of acute deterioration (e.g., new focal neurological deficit, seizure, or sudden drop in the patient's level of consciousness)
BP via arterial line continuously
Maintaining a stable systolic BP is important. BP instability occurs due to the loss of normal cerebral auto-regulation as a result of the acute brain injury
BP can also fluctuate after nimodipine and should be monitored to prevent hypotension and decreased cerebral perfusion[39]
ECG continuously
Consult immediately with a cardiologist about any ECG changes. Common cardiac findings in SAH include:
Patients with SAH may have ECG changes that mimic acute coronary syndrome and ST-elevation myocardial infarction. Seek specialist advice when deciding whether to perform a CT head scan before or after coronary angiography. Use your clinical judgement alongside specialist input to weigh up the likelihood of the patient having SAH versus acute coronary syndrome[85]
Aneurysm rupture can lead to cardiac complications, such as left ventricular subendocardial injury and takotsubo cardiomyopathy (even in the absence of acute coronary disease) leading to treatment delays.[39] SAH can also cause cardiac arrest[86]
Temperature continuously
Initial supportive care
Minimise the risk of rebleeding and vasospasm until the aneurysm is secured.[39]
Advise bed rest.
Provide analgesia in conscious patients.
Consider a stool softener and an anti-emetic in conscious patients.
Constipation is commonly seen in people with SAH, especially those who have been immobile as a result or treated with opioid analgesics.
Seek advice from a haematologist for urgent drug-specific reversal strategies.
Stop and where possible reverse all anticoagulation (e.g., warfarin, direct oral anticoagulants). Stop all antiplatelet agents; platelet transfusions are not usually necessary.
Maintain normovolaemia and avoid hypovolaemia; this is a risk factor for ischaemic complications.
Avoid hypotension and hyponatraemia.
Stop any antihypertensive medication and do not treat hypertension unless it is extreme.[39]
Maintain systolic blood pressure <180 mmHg until occlusion of aneurysm.[39]
Treat moderate to severe hyponatraemia (sodium levels <131 mmol/L [<131 mEq/L]) with hypertonic saline 3%.[115][120] Hyponatraemia is the most common electrolyte imbalance in SAH.[39]
Use compression stockings and intermittent compression by pneumatic devices in high-risk patients before occlusion of the aneurysm for the prevention of deep vein thrombosis and pulmonary embolism.[39]
Do not routinely give anticonvulsants to prevent seizures in patients with SAH.[39]
Aneurysm rupture itself can cause stress hyperglycaemia. Follow your local protocol in case of hyperglycaemia.[39]
Treatment of neurological complications
Rebleeding
Be aware that the risk of rebleeding is highest within 24 hours of the onset of symptoms.[37]
If you suspect a rebleed, consult with a neurosurgeon and arrange an urgent non-contrast CT head rescan.
Antifibrinolytic agents are not routinely recommended.[39] Short-course tranexamic acid is occasionally used in practice if interventional treatment to secure the aneurysm is suitable but not available within a short time frame. However, administration of tranexamic acid should not delay interventional treatment to secure the aneurysm.[37]
Acute hydrocephalus
If you suspect hydrocephalus, consult with a neurosurgeon and arrange an urgent non-contrast CT head rescan. Acute hydrocephalus can lead to severe disability or death if not treated promptly.[37][121]
The neurosurgeon may consider drainage or diversion of cerebrospinal fluid for a patient with neurological deterioration caused by acute hydrocephalus.[37][122]
Seizures
Consult immediately with a neurologist or a neurosurgeon if the patient has clinically apparent seizures. The choice of anticonvulsant will depend on the patient characteristics.[39]
Vasospasm and delayed cerebral ischaemia
Check for the development of new neurological symptoms/signs that may indicate cerebral ischaemia and consult with a neurosurgeon immediately for advice on treatment. Symptomatic vasospasm and delayed cerebral ischaemia (DCI) often develops 72 hours after securing the aneurysm.
Clinical pointers to the presence of vasospasm/DCI:
A drop in GCS score of 2 or more
A new focal neurological deficit (e.g., unilateral motor or sensory loss, speech disturbance, or visual fields loss) not attributable to rebleeding, hydrocephalus, hyponatraemia, seizures, or any other cause.
Current available treatments such as triple-H (uses hypertension, hypervolaemia, and haemodilution) or endovascular strategies (e.g., balloon angioplasty or intra-arterial vasodilators) aren’t supported by good-quality clinical trials. Hypertensive therapy alone is, however, still widely practiced.
SAH is an acute life-threatening condition.[37] If interventional treatment is planned, this should be carried out at the earliest opportunity to prevent rebleeding. The risk of rebleeding is highest within 24 hours of the onset of symptoms. Therefore, it is essential to recognise the condition quickly and be aware of the spectrum of presentation.[42]
Patients with SAH are at risk of haemodynamic instability and neurological deterioration.
Main goals of treatment are to:
Stabilise the patient
Determine the patient’s prognosis not only from the haemorrhage itself but also resulting from treatment of the aneurysm
Secure the aneurysm within 48 hours
Prevent and manage complications pre- or post-occlusion of aneurysm, particularly rebleeding and cerebral vasospasm
Aneurysm rupture can cause stress hyperglycaemia, cardiopulmonary complications, and increased blood coagulability.[39]
Practical tip
Stabilise and investigate the patient at the same time to allow for timely treatment. Securing the aneurysm early eliminates the risk of rebleeding, and ensures vasospasm and other complications can be treated aggressively.[39]
As soon as the diagnosis of SAH is confirmed, urgently discuss with a specialist neurosurgical neurosciences centre the need for transfer of care of the patient to the specialist centre.[37]
Do not use a SAH severity score in isolation to determine the need for, or timing of, transfer of care to a specialist neurosurgical centre.[37][49]
Patients with Glasgow Coma Scale (GCS) score ≤8 or falling
Use an ABC approach.
Provide airway protection with simple airway manoeuvres and adjuncts (e.g., oropharyngeal or nasopharyngeal airways, as appropriate).
Monitor controlled oxygen therapy. An upper SpO2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. Evidence suggests that liberal use of supplemental oxygen (target SpO2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[45] A lower target SpO2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[46]
Call the anaesthetist. These patients require intubation, deep sedation, and sometimes paralysis. Involving an anaesthetist is standard practice in the UK. However, in some countries, urgent assessment, intubation, and sedation is performed by a trained team in the emergency department.
Cerebroprotective induction is required to protect against laryngoscopy-induced increases in intracranial pressure.
Give fluid resuscitation to restore normovolaemia.
Start with 3 L/day (isotonic/normal saline 0.9%); adjust infusion for oral intake and supplement other electrolytes as necessary.[39]
Avoid all hypotonic fluids.
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in acutely ill adults who are not at risk of hypercapnia.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO2 range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[46][125]
The Global Initiative For Asthma (GINA) guidelines recommend a target SpO2 range of 93% to 96% in the context of a severe exacerbation of acute asthma.[126]
One systematic review including a meta-analysis of data from 25 randomised controlled trials, published in 2018, found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[45] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy versus conservative therapy group (95% CI 2 to 22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (RR 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, or cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, or patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO2 limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, or sickle cell crisis).[127]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[128]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[129] The BTS guidance is due for a review in 2022.
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[130][131][132]
Monitor neurological status.
Use the Glasgow Coma Scale (serial) but be aware that the need for monitoring clinically neurological status in these patients must be balanced against the risk of harm from an unsecured aneurysm or inadequate spontaneous ventilation. If in doubt, consult with an anaesthetist and a neurosurgeon. [ Glasgow Coma Scale Opens in new window ]
When conducting a neurological assessment, check the patient’s care record and if opioid analgesia has been given, take into account its sedating and pupillary effects.[37]
Check pupils for size, shape, and reactivity to light every 20 minutes once the patient is sedated and paralysed. Discuss any pupil changes with a neurosurgeon.
Fixed and dilated pupils (especially if bilateral) in comatose patients are associated with poor prognosis.[47] Consider immediate hypertonic saline or mannitol.
Practical tip
A reduced level of consciousness and seizures on presentation are risk factors for aspiration pneumonia.
Practical tip
Look for hydrocephalus on the computed tomography scan. This may explain decreased level of consciousness.
Monitor ECG continuously for arrhythmias that could cause haemodynamic instability.
Prevention of delayed cerebral ischaemia
Consider enteral nimodipine for all patients with confirmed SAH (if not contraindicated).[37] In practice, the decision to start nimodipine should be made by a neurosurgeon; if a neurosurgeon is not available, a critical care specialist should make this decision. If used, nimodipine should be given for 14 to 21 days.[39][40]
Only use intravenous nimodipine within a specialist setting and if enteral treatment is not suitable.[37][133]
If nimodipine is potentially contraindicated (e.g., after recent myocardial infarction), seek specialist advice.
Nimodipine is a dihydropyridine calcium-channel blocker that relaxes and widens blood vessels. Limited evidence shows some reductions in mortality, rebleeding, disability, and delayed cerebral ischaemia with nimodipine.[37][133] Although nimodipine is widely used in practice, there is uncertainty about its benefits owing to the lack of compelling contemporary data.[37][133] Nimodipine was initially investigated for the prevention of vasospasm; however, despite its vasodilatory effects on cerebral blood vessels, it is unclear whether nimodipine affects the incidence of angiographic or symptomatic vasospasm.[39][134][135][136][137][138]
Practical tip
Monitor BP after giving nimodipine to prevent hypotension and decreased cerebral perfusion.[39] Nimodipine is a potent cerebral vasodilator and may cause systemic hypotension in some patients.
Evidence: Nimodipine use and route of administration
Nimpodipine may reduce mortality, rebleeding, disability, and delayed cerebral ischaemia in people with SAH. However, this effect is uncertain and based on early evidence, before current best practice in securing ruptured aneurysms. Intravenous nimodipine is unlikely to be cost-effective in most situations. Therefore, administration should be via the enteral route where possible.
The UK National Institute for Health and Care Excellence (NICE) advises to consider enteral nimodipine (oral or via nasogastric tube) for people with a confirmed subarachnoid haemorrhage and to only use the intravenous route in specialist settings if enteral treatment is unsuitable (e.g., patients with poor drug absorption).[37][133]
Limited evidence showed some reduction in mortality, rebleeding, disability, and delayed cerebral ischaemia with nimodipine compared with placebo (7 randomised controlled trials [RCTs]) or no nimodipine (1 RCT).
Most of the evidence was assessed by NICE as low or very low quality using the GRADE approach, with wide confidence intervals meaning there was uncertainty about the clinical significance of many results.
All included studies were conducted in the 1980s. Since then there have been significant changes in neurosurgical management, raising some concerns about the applicability to current clinical practice.
Four studies included intravenous nimodipine. Intravenous nimodipine is expensive and only used in an intensive care setting. The guideline committee felt it was unlikely to be cost-effective in most patients, including those who are unconscious, ventilated, or otherwise unable to swallow, where the enteral route via a nasogastric tube is still preferable.
As there is no evidence of significant harms with nimodipine, and there remains a potential benefit, NICE made a weak recommendation to consider using nimodipine, while also making a research recommendation to evaluate whether nimodipine still has a role in the treatment of SAH.
Observe patient continuously at least until occlusion of the aneurysm.[39]
Practical tip
The frequency of observations may be reduced after aneurysm treatment, but must continue regularly as vasospasm and delayed cerebral ischaemia often occurs after 72 hours of securing the aneurysm.
Neurological status (GCS) and neurological examination
Use the Glasgow Coma Scale (GCS) and check neurological status at least every hour until occlusion of aneurysm. [ Glasgow Coma Scale Opens in new window ] [39]
Practical tip
A poor neurological status on admission seems to predict cardiac abnormalities, which are thought to be secondary to overwhelming sympathetic activation.[56][57][58][59]
Reduced awareness and seizures on presentation are risk factors for aspiration pneumonia. See Aspiration pneumonia.
Perform a gross neurological examination and observe for signs of deterioration (e.g., new focal neurological deficit, seizure, or sudden drop in the patient's level of consciousness).[40]
A unilateral fixed pupil with other signs of oculomotor nerve palsy in a conscious patient is more likely to represent a ruptured posterior communicating artery aneurysm than raised intracranial pressure. This would not carry the same poor prognosis as pupillary changes in unconscious patients.
When conducting a neurological assessment, check the patient’s care record and if opioid analgesia has been given, take into account its sedating and pupillary effects.[37]
If patient with GCS score ≥9 deteriorates to GCS score ≤8 then follow recommendations for GCS score ≤8 or falling and consult with a neurosurgeon immediately. See Urgent initial management above on patients with GCS score ≤8 or falling.
Practical tip
While overlapping features can make it hard to clinically differentiate the neurological complications of SAH, the following may provide some clues before the aneurysm is secured.
Think rebleed if there:
Is a sudden drop in conscious level
Is a spike in blood pressure
Is tonic/extensor posturing
Are pupillary changes.
Think hydrocephalus if there is:
A gradually worsening level of arousal with relative preservation of deliberate motor responses ± severe headache/vomiting/agitation.
Think vasospasm if there are:
New focal neurological signs or symptoms occurring approximately 3 to 14 days after SAH.
Blood pressure
Monitor blood pressure (BP) continuously via an arterial line.[39]
Maintaining a stable systolic BP is important. Blood pressure instability occurs due to the loss of normal cerebral auto-regulation as a result of the acute brain injury.
Blood pressure can also fluctuate after nimodipine and should be monitored to prevent hypotension and decreased cerebral perfusion.[39]
In line with recommendations from the European Stroke Organisation:[39]
Maintain systolic blood pressure (SBP) <180 mmHg until occlusion of the aneurysm to reduce the risk of bleeding[39]
If SBP remains high, consider further lowering of blood pressure
Maintain the mean arterial pressure at least >90 mmHg if BP is lowered
Stop any antihypertensive medication
Do not treat hypertension unless it is extreme
Limits for extreme blood pressures should be set on an individual basis taking into account the age of the patient, pre-SAH blood pressures, and cardiac history.
Practical tip
A reduction in BP may be already achieved by giving nimodipine (to prevent delayed cerebral ischaemia) and analgesia.[39] Nimodipine is a potent cerebral vasodilator and may cause systemic hypotension in some patients. Elevated BP following SAH is often due to pain, anxiety, and generalised sympathetic activation.
ECG
Consult immediately with a cardiologist if you see any ECG changes.
Common cardiac findings in SAH include:
Practical tip
Patients with SAH may have ECG changes that mimic acute coronary syndrome and ST-elevation myocardial infarction. Seek specialist advice when deciding whether to perform a computed tomography head scan before or after coronary angiography. Use your clinical judgement alongside specialist input to weigh up the likelihood of the patient having SAH versus acute coronary syndrome.[85]
Ventricular wall motion abnormalities may occur and an apex-sparing pattern is seen in some patients.
[Figure caption and citation for the preceding image starts]: ECG done on admission of a patient with subarachnoid haemorrhage; note peaked, tall T waves (1 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].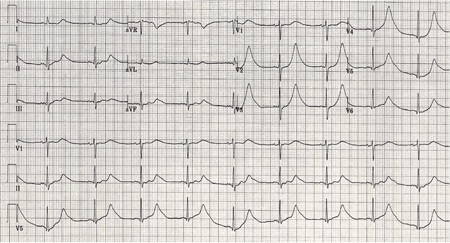 [Figure caption and citation for the preceding image starts]: Same patient, 24 hours later; note normalisation of T waves (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Same patient, 24 hours later; note normalisation of T waves (2 of 2)Courtesy of Dr Salah Keyrouz; used with permission [Citation ends].
Aneurysm rupture itself can cause cardiac complications, such as left ventricular subendocardial injury and takotsubo cardiomyopathy (even in the absence of acute coronary disease) leading to treatment delays.[39] SAH can also cause cardiac arrest.[86] Cardiac abnormalities in SAH may be due to massive catecholamine release resulting in overwhelming sympathetic activation.[56][57][58][59]
Temperature
Monitor temperature continuously.[39] Over half of patients with SAH have a fever, even after routine administration of paracetamol.[139] Increased temperature is an independent factor for poor outcome.[39]
Investigate any fever but be aware that no infection is found in around 20% of patients. Fever is thought to be due to inflammatory responses to extravasated blood in the subarachnoid space.[39]
Electrolytes and fluids
Give fluids, aiming for normovolaemia and normal electrolyte balance.
Maintaining normovolaemia is the standard of care in the treatment of SAH. This is to prevent cerebral vasospasm.
Start with 3 L/day (isotonic/normal saline 0.9%), and adjust infusion for oral intake and supplement other electrolytes as necessary.[39]
Avoid hypovolaemia; this is a risk factor for ischaemic complications.
Check for signs of hyponatraemia, which is the commonest electrolyte imbalance in SAH and is usually associated with syndrome of inappropriate antidiuretic hormone secretion (SIADH). Other causes include cerebral salt-wasting syndrome. Occurs in up to 50% of patients and it is associated with increased morbidity including vasospasm.[79][80]
Treat moderate to severe hyponatraemia (sodium levels <131 mmol/L [<131 mEq/L]) with hypertonic saline 3%.[115][120]
Monitor sodium levels for response frequently. Monitor the rate and composition of the hypertonic solution, fluid balance and adjust accordingly.
Aim for normovolaemia also in case of hyponatraemia.[39] The administration of isotonic saline can prevent volume contraction but not hyponatraemia.
Practical tip
Do not restrict fluids in patients with suspected SIADH in the first few weeks after SAH.
Practical tip
In patients with confirmed SAH, do not manage hyponatraemia with fluid restriction (despite restriction of fluids being a common treatment of hyponatraemia due to other aetiologies).
Analgesia
Provide sufficient analgesia for conscious patients to avoid elevated blood pressure and prevent rebleeding.
Pain is associated with a transient elevation in blood pressure and increased risk of rebleeding.
In line with recommendations from the European Stroke Organisation:[39]
Start with paracetamol. Avoid aspirin or non-steroidal anti-inflammatory drugs before aneurysm occlusion
Use codeine or tramadol for severe pain. If the patient is still in pain, use morphine or oxycodone.
Document administration of opioid analgesia in the patient’s healthcare record.[37] Opioid analgesia may affect neurological assessment given its sedating and pupillary effects.[37]
Fever
Give routine antipyretic medication (e.g., paracetamol) and apply cooling blankets to aim for normothermia.[39] Only give an antipyretic medication if an analgesic without antipyretic properties (e.g., opioid) is being used. If paracetamol is being used as an analgesic, this is also a suitable antipyretic.
Prevent Valsalva manoeuvres
In addition to analgesia and sedation, other recommended measures to prevent rebleeding before occlusion of the aneurysm are:[39]
Advise bed rest
Give a stool softener (e.g., docusate, senna) and an anti-emetic to conscious patients. Constipation is commonly seen in people with SAH, especially those who have been immobile as a result or treated with opioid analgesics
These are given to prevent Valsalva manoeuvres with resultant peaks in systolic blood pressure and intracranial pressure.
Stop and reverse anticoagulation
Stop all anticoagulants and antiplatelet agents; platelet transfusions are not usually necessary.
Commonly used anticoagulants include warfarin or direct oral anticoagulants (e.g., dabigatran, apixaban, edoxaban, rivaroxaban).
Practical tip
When deciding how long to stop anticoagulants for, take into account the anticipated need for further invasive procedures as well as the indication for anticoagulation.
Seek advice from a haematologist for urgent drug-specific reversal strategies.
Check the international normalised ratio (INR) and reverse anticoagulant effect immediately. This is to reduce further bleeding.
Hyperglycaemia
Check for signs of hyperglycaemia, a common complication of acute brain injury (from rupture of the aneurysm).[39]
Follow local protocols. European guidelines recommend treating hyperglycaemia over 10 mmol/L.[39]
Prevent deep venous thrombosis and pulmonary embolism
Consider compression stockings and intermittent compression by pneumatic devices in high-risk patients before occlusion of the aneurysm.[39]
Consider adding a low molecular weight heparin (LMWH) after securing the aneurysm, at least 12 hours after surgical clipping and immediately after coiling.[39] In practice in the UK, prescription of LMWH will be prompted if appropriate once you have recorded your venous thromboembolism risk assessment in the patient’s electronic record.
Electroencephalogram (EEG)
Order a standard EEG if you suspect non-convulsive status epilepticus. See Status epilepticus.
Do not perform routine continuous EEG monitoring in patients with SAH.[39][114]
An interventional neuroradiologist and a neurosurgeon should decide the best mode of intervention, taking into account the patient's clinical condition, the characteristics of the aneurysm, and the amount and location of subarachnoid blood.[37][39] A proposed treatment plan should be documented, in discussion with the patient, and their family or carers if appropriate, based on the following options:[37]
Interventional treatment with endovascular coiling
This is the treatment of choice in ruptured aneurysms that can be equally effectively treated with either coiling or clipping.[37][39][140] [
 ]
[Evidence B]
]
[Evidence B]
Interventional treatment with neurosurgical clipping
No interventional procedure, with monitoring to check for clinical improvement and reassess the options for treatment
Interventional treatment is not suitable for some patients with aneurysmal SAH, including those whose clinical condition is poor (e.g., patients with severe neurological deficit, impaired consciousness, or requirement for ventilatory support).[141]
Practical tip
Older patients should not be excluded from treatment of the aneurysm based solely on their age. The decision to treat actively should take into account the patient’s clinical condition.[39]
Do not use a SAH severity score in isolation to determine the suitability of any management option.[37]
Practical tip
In practice, a neurosurgeon might grade the severity of initial bleed by using:[39]
The Modified Hunt and Hess classification for patients with SAH, or
The World Federation of Neurological Surgeons (WFNS) Grading System for Subarachnoid Hemorrhage scale.
Poor SAH grades (i.e., high grades) on admission are associated with higher risk of rebleeding, complications, and mortality.[142][143]
For the specifics of the Hunt and Hess and WFNS grading scales see Criteria.
Evidence: Timing of intervention to secure aneurysm
If interventional treatment (endovascular coiling or neurosurgical clipping) of the culprit aneurysm is planned it should be done as soon as possible to reduce the risk of rebleeding.
The UK National Institute for Health and Care Excellence (NICE) recommends that if interventional treatment is planned it is carried out at the earliest opportunity to prevent rebleeding.[37][144]
NICE noted that around half of patients have a second bleed from the culprit aneurysm within weeks, with the highest risk being in the first 24 hours, and that the mortality from a rebleed can be >50%.
Two randomised controlled trials (RCTs) and 12 observational studies were included in the NICE evidence review.
The included studies compared early (defined variably as ≤24 hours, ≤48 hours, or ≤72 hours) with delayed intervention.
The majority of the evidence for each outcome was assessed by NICE as very low quality using the GRADE approach.
The RCT evidence was limited as one was from 1989 and the other was very small (n=8).
The guideline committee felt it could not make a definitive recommendation on timing of intervention, but made a consensus recommendation that treatment should be carried out as soon as possible.
Overall the evidence base was insufficient in quality and quantity.
Observational studies were at risk of selection bias with patients expected to do better receiving earlier treatment.
The NICE guideline states that most interventions in the UK are carried out within 48 hours of admission; therefore, the committee did not expect its recommendation to have much effect on current practice.
European guidelines published in 2012 recommend to intervene as early as possible to reduce the risk of rebleeding and definitely within 72 hours after onset of first symptoms.[39]
The UK Royal College of Physicians guidelines (updated in 2023) recommend to intervene within 48 hours of onset of symptoms for good-grade patients (Hunt and Hess or World Federation of Neurological Sciences grades 1-3), or within a maximum of 48 hours of diagnosis if presentation was delayed.[40]
Interventional treatment
If interventional treatment via endovascular coiling or neurosurgical clipping is planned, this should be carried out at the earliest opportunity to prevent rebleeding. The risk of rebleeding is highest within 24 hours of the onset of symptoms.[37]
Any decisions on how to manage the culprit aneurysm should be interdisciplinary, based on the experience of the neurosurgeon and the interventional neuroradiologist, the clinical condition of the patient, the characteristics of the aneurysm, and the amount and location of subarachnoid blood.[37]
The UK National Institute for Health and Care Excellence (NICE) recommends endovascular coiling as first choice, because it is less invasive and potentially safer than neurosurgical clipping. Neurosurgical clipping should be considered if endovascular coiling is not suitable.[37]
Specific factors to consider include:[39]
Patient
Age
Comorbidity
Presence of intracerebral haemorrhage
SAH grade (surgical risk assessment): poor-grade versus good-grade
Aneurysm size, location, and shape
Status of collaterals (collateral circulation refers to the secondary network of vascular channels that stabilises cerebral blood flow when principal channels fail)
Procedure: technical skills and availability of the neurosurgeon and the endovascular radiologist
Logistics: availability of multidisciplinary team.
Factors in favour of surgical clipping and endovascular coiling[39]
Age | Age ≤70 years | Age >70 years |
Space-occupying intracerebral haemorrhage | Present | Not present |
Location of aneurysm | Middle cerebral artery and pericallosal aneurysm | Posterior location |
Neck of aneurysm | Wide-necked or fusiform | Small neck and saccular |
Shape of aneurysm | Arterial branches exiting directly out of the aneurysmal sack Other unfavourable vascular and aneurysmal shape for coiling | Unilobar shape |
For example, coiling is favoured in older people as recovery from craniotomy takes longer and carries more complications in this population. A coil in a wide-neck aneurysm may just slip out again so clipping is favoured in wide-neck aneurysms. Neck recurrence and rebleeding are less frequent in the long term following clipping; therefore, surgery may offer a more definitive solution to younger patients.
Complications associated with each procedure
Surgical clipping | Endovascular coiling |
|
|
Evidence: Endovascular coiling versus surgical clipping
Endovascular coiling is associated with a better outcome (i.e., more people survive and are independent in their daily living) than surgical clipping in people in good clinical condition with aneurysms suitable for both interventions. However, the risk of post-procedure rebleeding is higher in people treated with coiling.
A Cochrane review of four randomised trials involving 2458 patients found:[140]
[  ]
[Evidence B]
]
[Evidence B]
The relative risk (RR) of poor outcome (death or dependency at 12 months) for endovascular coiling versus neurosurgical clipping was 0.77 (95% CI 0.67 to 0.87; 2429 participants) and the absolute risk reduction was 7% (95% CI 4% to 11%)
The RR of delayed cerebral ischaemia at 2 to 3 months for endovascular coiling versus neurosurgical clipping was 0.84 (95% CI 0.74 to 0.96; 2450 participants)
The RR of post-procedure rebleeding for endovascular coiling versus neurosurgical clipping was 1.83 (95% CI 1.04 to 3.23; 4 trials, 2458 participants) at 1 year, and 2.69 (95% CI 1.50 to 4.81; 1 trial, 1323 participants) at 10 years
Complication rates were similar among groups.
An 18-year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT) found:[148]
Patients undergoing endovascular coiling were significantly more likely to be alive and independent at 10 years than patients undergoing surgical clipping (odds ratio 1.34; 95% CI 1.07 to 1.67)
A total of 33 patients had a recurrent SAH more than 1 year after their initial haemorrhage (17 from the target aneurysm). Rebleeding was more likely after endovascular coiling than after neurosurgical clipping, but the risk was small
In both groups, the risk of death and rebleeding from the treated aneurysm up to 18 years was very small and similar to the risk of recurrent haemorrhage from another aneurysm.
Unruptured intracranial aneurysms
Asymptomatic unruptured intracranial aneurysms may be found after SAH as additional aneurysms (i.e., they are not the source of the bleed). In these patients, the ruptured aneurysm should be treated first.[39] The decision whether to treat subsequent unruptured aneurysms depends on:[39]
Aneurysmal factors such as size (the larger the aneurysm, the higher the chance of rupture), and location
The estimated lifetime risk of the aneurysm rupturing[37]
Procedural risk (range of 5% to 50% vs. spontaneous rupture risk 0% to 10%, per year) and benefit (life expectancy with or without minor deficit)[39]
Comorbidities[37]
Patient preference.[37]
Any decisions on how to manage the culprit aneurysm should be interdisciplinary, based on the experience of the neurosurgeon and the interventional neuroradiologist, and on the clinical condition of the patient, the characteristics of the aneurysm, and the amount and location of subarachnoid blood.[37][39]
Interventional treatment is not suitable for some patients with aneurysmal SAH, including those whose clinical condition is poor (e.g., patients with severe neurological deficit, impaired consciousness, or requirement for ventilatory support).[141] If an interventional procedure is not deemed to be suitable, the patient should be monitored for clinical improvement and the options for treatment reassessed as appropriate.[37]
For people with unexplained neurological deterioration after a subarachnoid haemorrhage, a non-contrast CT head scan should be the first diagnostic investigation to determine the cause.[37]
Do not use transcranial doppler monitoring to guide clinical management of an aneurysmal subarachnoid haemorrhage (except in the context of clinical research).[37]
Rebleeding
Be aware that the risk of rebleeding is highest within 24 hours of the onset of symptoms.[37]
If you suspect a rebleed, consult with a neurosurgeon and arrange an urgent non-contrast CT head rescan.
Early aneurysm repair (by endovascular coiling or surgical clipping) is the only effective intervention to prevent rebleeding and reduce mortality.[39][145][149][150][151][152][153][154][155][156][157][158]
Antifibrinolytic agents (e.g., tranexamic acid) are not routinely recommended.[39] Short-course tranexamic acid is occasionally used in practice if interventional treatment to secure the aneurysm is suitable but not available within a short time frame. However, administration of tranexamic acid should not delay interventional treatment to secure the aneurysm.[37]
Evidence on tranexamic acid in the management of SAH is mixed; most data are from small studies conducted in the 1970s and 1980s. Some of the evidence suggests that short courses of intravenous tranexamic acid started immediately after diagnosis and before a planned intervention (endovascular coiling or neurosurgical clipping) may reduce the risks of rebleeding. However, there is no evidence showing that tranexamic acid reduces death or disability.[37][159] [
 ]
]
Practical tip
While overlapping features can make it hard to clinically differentiate the neurological complications of SAH, the following may provide some clues before the aneurysm is secured.
Think rebleed if there:
Is a sudden drop in conscious level
Is a spike in blood pressure
Is tonic/extensor posturing
Are pupillary changes.
Rebleeding is the most severe early complication of SAH with a reported incidence of up to 15% in the first 24 hours, and a mortality rate of approximately 70%.[160][161][162]
A rebleed may substantially alter the prognosis and may even influence the decision whether to treat actively.
Evidence: Antifibrinolytics in preventing rebleeding in SAH
Antifibrinolytic treatment is associated with a reduced risk of rebleeding, but it is not associated with improved survival or the chance of being independent in everyday activities, and increases the risk of cerebral ischaemia.
A Cochrane review of 10 randomised trials involving 1904 participants comparing oral or intravenous antifibrinolytic drugs (tranexamic acid, epsilon amino-caproic acid, or an equivalent) with control in people with SAH of suspected or proven aneurysmal cause found:[159]
The pooled relative risk (RR) for poor outcome (death, vegetative state, or severe disability) was 1.02 (95% CI 0.9 to 1.15). The pooled RR for death from all causes was 1.00 (95% CI 0.85 to 1.18).
The pooled RR for reported cerebral ischaemia was 1.41 (95% CI 1.04 to 1.91; 83 per 1000 participants), again with heterogeneity between the trials.
No effect on the reported rate of hydrocephalus was observed with antifibrinolytic therapy (RR 1.11, 95% CI 0.90 to 1.36).
At the end of the follow-up period (at least 3 months after SAH), the RR for rebleeding was 0.65 (95% CI 0.44 to 0.97; 78 per 1000 participants), but there was heterogeneity between the trials.
In one trial that combined short-term antifibrinolytic treatment (<72 hours) with preventative measures for cerebral ischaemia, the RR for people receiving antifibrinolytic treatment (<72 hours) for ischaemia prevention was 0.85 (95% CI 0.64 to 1.14).
Acute hydrocephalus
If you suspect hydrocephalus, consult with a neurosurgeon and arrange an urgent non-contrast CT head rescan. Acute hydrocephalus can lead to severe disability or death if not treated promptly.[37][121]
The neurosurgeon may consider drainage or diversion of cerebrospinal fluid for a patient with neurological deterioration caused by acute hydrocephalus.[37][122]
Either drainage or diversion could be considered. There is no evidence on the effectiveness of different techniques for drainage or diversion in acute hydrocephalus.[37]
Practical tip
While overlapping features can make it hard to clinically differentiate the neurological complications of SAH, the following may provide some clues before the aneurysm is secured.
Think hydrocephalus if there is:
A gradually worsening level of arousal with relative preservation of deliberate motor responses ± severe headache/vomiting/agitation.
Acute symptomatic obstructive hydrocephalus occurs in 15% to 20% of patients during the first 72 hours.[10][11][121] It occurs due to the presence of intraventricular blood and, to a lesser extent, thick cisternal blood collection.[121][163]
Seizures
Consult immediately with a neurologist or a neurosurgeon if the patient has clinically apparent seizures. The choice of anticonvulsant will depend on the patient characteristics.[39]
Follow your hospital protocol. Levetiracetam and sodium valproate are commonly used.
Do not routinely give prophylactic anticonvulsants.[39]
In selected patients, such as those with a large intracerebral haemorrhage, prophylactic anticonvulsants may be considered. Consult with the neurosurgeon.
Evidence: Prophylactic anticonvulsant treatment
There is a lack of evidence to support the routine use of prophylactic anticonvulsants in patients with SAH.
An analysis of data from 3552 patients from four prospective, randomised, double-blind, placebo-controlled trials showed worse outcomes (i.e., cerebral ischaemia, neurological deterioration, cerebral infarction, and elevated temperature during hospitalisation) in those receiving prophylactic anticonvulsants (65%) compared with those who did not (35%).[164]
Another study showed that phenytoin was associated with functional and cognitive disability when used prophylactically after SAH. Phenytoin was associated with poor functional outcome at 14 days (odds ratio [OR] 1.5 per quartile, 95% CI 1.3 to 1.8, P <0.001), although not at 3 months (P = 0.09). The effect remained (OR 1.6 per quartile, 95% CI 1.2 to 2.1, P <0.001) after correction for admission Glasgow Coma Scale score, fever, stroke, age, National Institutes of Health Stroke Scale ≥10, hydrocephalus, clinical vasospasm, and aneurysm rebleeding.[165]
A retrospective analysis of SAH patients admitted from 2005 to 2010 looked at the reduction in seizures with prophylactic anticonvulsants on admission. After 2007, patients did not receive anticonvulsant treatment for the prevention of seizures. The results showed that anticonvulsants were not significantly associated with a reduction in the risk of seizure when use d prophylactically compared with when they were not (11% vs. 8%, respectively; P = 0.33).[166]
A Cochrane review concluded that there is a lack of evidence to support or refute the use of anticonvulsant treatment for the prevention of seizures.[167]
Seizures at the onset of SAH occur in around 7% of patients.[60] About 10% develop seizures in the first few weeks.[61]
Convulsive status epilepticus is rare in SAH (0.2% of patients).[168]
Non-convulsive status epilepticus and subclinical seizures seem to contribute to prolonged impairment of consciousness in patients with SAH. However, non-convulsive status epilepticus can be a diagnosis of exclusion and routine continuous electroencephalographic monitoring in SAH is not recommended as it is labour-intensive, can be misinterpreted, and lacks proof of effectiveness.[39][114] See Status epilepticus .
Practical tip
Do not assume clinical deterioration is due to non-convulsive status epilepticus until other causes of deterioration have been excluded.
Vasospasm and delayed cerebral ischaemia (DCI)
The diagnosis is often challenging, particularly in patients who already have a neurological deficit or in those who are sedated. Both vasospasm and DCI may be asymptomatic. A timely diagnosis of DCI is essential to start treatment early and prevent irreversible neurological deficits and death.[39]
Check for the development of new neurological symptoms/signs that may indicate vasospasm/DCI and consult with a neurosurgeon immediately for advice on treatment.
Clinical pointers to the presence of vasospasm/DCI:
A drop in Glasgow Coma Scale score of 2 or more
A new focal neurological deficit (e.g., unilateral motor or sensory loss, speech disturbance, or visual fields loss) occurring 3 to 14 days after SAH, not attributable to rebleeding, hydrocephalus, hyponatraemia, seizures, or any other cause.
Practical tip
Monitoring should continue beyond 72 hours in patients with SAH for the occurrence of vasospasm and DCI.
Vasospasm is a delayed, focal, or diffuse narrowing of large capacitance vessels of the circle of Willis. It accounts for 23% of deaths related to SAH.[84]
Vasospasm (as seen on angiography) develops between days 3 and 14 after SAH in up to 70% of patients and, of these, around 30% have clinical symptoms.
Half of those with vasospasm develop DCI secondary to reduced regional or overall cerebral blood flow.[137][169]
Consult with the radiologist or neurosurgeon and follow your local protocol for the radiological diagnosis of clinical signs of delayed cerebral ischaemia or asymptomatic vasospasm.
Computed tomography angiography, digital subtraction angiography, or computed tomography perfusion imaging may be used.[170]
Vasospasm-related ischaemia may become symptomatic despite giving nimodipine prophylactically.
Ensure euvolaemia (normal blood volume) in patients with delayed cerebral ischaemia after an aneurysmal SAH. Intravenous fluid is usually given to ensure euvolemia; if symptoms persist, a vasopressor can be administered to raise systemic blood pressure. Bear in mind that clinical improvement after these measures may be temporary, and there is no evidence of impact on longer-term outcomes.[37]
Current available treatments such as triple-H, endovascular strategies (e.g., balloon angioplasty or intra-arterial vasodilators), or hypertensive therapy aren’t supported by good-quality clinical trials. However, hypertensive therapy alone is still widely practiced.
[Figure caption and citation for the preceding image starts]: Severe vasospasm of distal left internal carotid artery and proximal middle and anterior cerebral arteries before (A) and after (B) intra-arterial infusion of nicardipine and transluminal balloon angioplastyCourtesy of Dr Salah Keyrouz; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Left frontal infarct (arrows) in a patient with subarachnoid haemorrhage-related vasospasmCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Left frontal infarct (arrows) in a patient with subarachnoid haemorrhage-related vasospasmCourtesy of Dr Salah Keyrouz; used with permission [Citation ends].
Triple-H therapy
Triple-H therapy uses hypertension, hypervolaemia, and haemodilution to improve cerebral blood flow and oxygen delivery in symptomatic vasospasm (following occlusion of aneurysm).
The use of triple-H therapy remains controversial and practice varies widely. Seek specialist advice.
European guidelines do not recommend its use.[39] This is based on the lack of evidence from controlled studies for induced hypertension or hypervolaemia to improve outcomes in patients with DCI, and the reported risks of deliberately increasing arterial pressure and plasma volume, which include increased cerebral oedema, haemorrhagic transformation in areas of infarction, reversible leukoencephalopathy, myocardial infarction, and congestive heart failure.[39][171][172]
Evidence: Efficacy of triple-H therapy
There is no good evidence from controlled trials that triple-H or its components improve cerebral blood flow (CBF) in SAH.
A systematic review of 11 studies (4 to 51 patients per study) found:[173]
Haemodilution alone did not change CBF
One of seven studies on hypervolaemia showed statistically significant CBF increase compared with baseline but there was no control group
Two of four studies using induced hypertension and one of two studies using triple-H showed significant CBF increase, but none used a control group
In uncontrolled studies, induced hypertension seemed to be more effective in increasing CBF than haemodilution or hypervolaemia.
Endovascular treatments
Endovascular strategies such as balloon angioplasty and intra-arterial injection of vasodilators (e.g., nimodipine) are recommended for refractory vasospasm.[34] However, there are no good-quality clinical trials demonstrating the efficacy of either approach.[174]
Practical tip
Perimesencephalic SAH is defined by exclusion of an aneurysmatic bleed and typical location of blood within the perimesencephalic and prepontine cisterns (i.e., no blood in sylvian and interhemispheric fissure).[52][113]
Although this topic does not cover PMSAH, it is worth bearing in mind these important considerations for confirmed PMSAH:
Do not give nimodipine (or other calcium-channel blockers) to people with PMSAH.[39] Vasospasm and delayed cerebral ischaemia (DCI) are rare in PMSAH.[175][176][177] More evidence is needed to understand the role of DCI in PMSAH[39]
Treat complications like hydrocephalus, hyponatraemia, and electrographic changes in the same way as recommended for SAH.[39] These may be as frequent in PMSAH as they are in aneurysmal SAH.[178]
Use of this content is subject to our disclaimer