Approach
For health-care providers, the diagnosis of sudden cardiac arrest is a definitive one. The patient is unresponsive, and assessment of airway, breathing, and circulation shows absence of normal breathing and no signs of circulation. Agonal respirations may be present. Assessment of the specific cardiac rhythm disturbance responsible should be immediate, whether by automated external defibrillator or other cardiac monitoring, and is essential for management (but should not delay CPR). Possibilities include ventricular fibrillation (VF), pulseless ventricular tachycardia (VT), pulseless electrical activity (PEA), and asystole. Torsades de pointes is a sub-group of polymorphic VT in patients with an underlying prolonged QT interval. [Figure caption and citation for the preceding image starts]: Monomorphic ventricular tachycardiaFrom the personal collections of Dr A. Askari and Dr A. Krishnaswamy; used with permission [Citation ends]. [Figure caption and citation for the preceding image starts]: Torsades de pointesFrom the personal collection of Dr A. Askari; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Torsades de pointesFrom the personal collection of Dr A. Askari; used with permission [Citation ends].
Lay rescuers are not able to determine with accuracy if a patient in cardiac arrest has a pulse. It is recommended that the lay rescuer assume that a person who is unresponsive and has absent or agonal respirations is in cardiac arrest and should begin CPR.[1] The risk of harm is low if the person is not in cardiac arrest.[1] Unfortunately, the rate of lay responders providing CPR in cardiac arrest is low (approximately 35% to 40% globally), despite the fact that lay responder CPR approximately doubles survival from out-of-hospital cardiac arrest (OHCA) and forms a crucial link in the chain of survival.[48]
Clinical assessment
A thorough but timely search must be made for the underlying cause of cardiac arrest, both during resuscitation and following successful resuscitation. Physical examination may demonstrate an elevated jugular venous pulse with decompensated heart failure. A history of trauma should prompt suspicion of cardiac tamponade, valvular disturbance, myocardial puncture, haemorrhage, or tension pneumothorax. Tracheal deviation suggests tension pneumothorax. A murmur may indicate a valvular cause of compromise, which might lead to sudden cardiac arrest. Pulmonary auscultation may reveal bilateral crepitations in decompensated heart failure, absent air entry in pneumothorax, or signs of underlying pulmonary disease. Neurological examination may reveal focal signs suggestive of intracranial pathology. Endotracheal intubation or a supraglottic airway device may be considered during resuscitation, particularly if there is difficulty achieving ventilation with a bag-mask device.
The patient’s medical, family, and medicine history should be reviewed at the earliest opportunity to identify possible causes. Clues that may be identified include:
Preceding symptoms: chest pain may indicate myocardial ischaemia or pulmonary embolism. Syncopal episodes may indicate structural heart disease or pre-existing arrhythmias. Palpitations may indicate pre-existing arrhythmias. One study found that among individuals with symptomatic OHCA, chest pain and dyspnoea in men, and dyspnoea in women, were the most common symptoms and had a moderate association with sudden cardiac arrest when compared with a control group of individuals who also called emergency services.[49]
Past medical history: potential causes include coronary artery disease (CAD) and its associated risk factors (e.g., hypertension, diabetes mellitus, smoking, hypercholesterolaemia, obesity), left ventricular dysfunction, hypertrophic cardiomyopathy, long QT syndrome, structural heart disease, use of illicit substances, medicines (including those that cause QT prolongation or diuretics that cause electrolyte disturbances), kidney disease (which may lead to hyperkalaemia), and history of eating disorders (which may contribute to hypokalaemia and/or hypophosphataemia).[43] Risk factors for deep vein thrombosis (DVT) and pulmonary embolism (PE) may increase the suspicion of PE as the aetiology; these include prior DVT/PE, unilateral calf swelling, active cancer, recent surgery, recent immobilisation, pregnancy, coagulopathy, or oral contraception use.
Family history of sudden cardiac arrest: may be due to familial QT-interval prolongation syndromes or familial cardiomyopathies. A family history of CAD may also be present.
Investigations
Several tests should be considered during cardiac arrest to assess the underlying cause and the condition of the patient:
Patients require continuous cardiac monitoring to assess rhythm.
An FBC should be performed to look for signs of haemorrhage (e.g., low haematocrit), which may cause hypovolaemia. An acute bleed may not show up, however, if haemodilution has not yet occurred.
Serum electrolytes should be checked for abnormalities, particularly hyper- or hypokalaemia; these may occur as either a cause or consequence of cardiac arrest.
Arterial blood gas (ABG) measurement is essential to assess acid-base status and may reveal respiratory acidosis, metabolic acidosis, respiratory acidosis with renal compensation, metabolic acidosis with respiratory compensation, mixed metabolic and respiratory acidosis, or hyperkalaemia. Respiratory and metabolic parameters should be optimised as necessary to normalise acid-base status. Abnormal results may be a result of sudden cardiac arrest itself rather than the underlying cause.
Cardiac biomarkers may be measured. However, elevations in markers of myocardial infarction (MI) may be a result of sudden cardiac arrest and do not necessarily mean that the underlying cause was an MI.
Point of care ultrasound (POCUS) may be used as an adjunct for patient evaluation during cardiac arrest, so long as it does not interfere with standard cardiac arrest treatments.[1][50] International Liaison Committee on Resuscitation guidelines recommend against its routine use, but suggest that if it can be performed by experienced staff without interrupting CPR, it can be used to look for a specific suspected reversible cause.[51] POCUS can be used to identify the presence or absence of cardiac activity, and can also identify features of cardiac tamponade, pneumothorax, haemorrhage, or pulmonary embolism. As these are potentially reversible causes of cardiac arrest, this could alter subsequent management. However, there is evidence that the use of POCUS in cardiac arrest prolongs the length of interruption of CPR during pulse checks.[51][52][53]
Further tests should be considered after achieving return of spontaneous circulation (ROSC):
An ECG should be performed immediately after ROSC, then subsequently to assess for evolving changes. Abnormalities that may be seen include prolonged QT interval, ST-segment or T-wave changes (indicating ischaemia or infarction in the event of ST-segment elevation), conduction abnormalities, ventricular hypertrophy, QRS prolongation in V1 through V3 and/or epsilon waves in cardiomyopathy, and T-wave inversion in V1 through V3 in arrhythmogenic right ventricular dysplasia (ARVD). Ambulatory ECG monitoring can be useful to capture sporadic events, either by continuous monitoring (Holter recording) over 24-48 hours, or using patient-activated ECG recorders (mobile health or smart phone technology) for infrequent events.[7]
Coronary angiography should be considered after achieving ROSC, as coronary disease is a predisposing factor for sudden cardiac arrest. In patients with ST-elevation MI (STEMI) on ECG, emergency coronary angiography, with or without percutaneous coronary intervention should be performed.[7][51][54] Emergency coronary angiography is also reasonable for select patients with suspected acute coronary syndrome without ST elevation, including those with haemodynamic/electrical instability or signs of ongoing ischaemia.[1] However, several randomised controlled trials in patients without signs of STEMI have shown no benefit in clinical outcomes for early coronary angiography, compared with delayed angiography.[7][55][56] International Liaison Committee on Resuscitation consensus recommendations suggest that in patients without signs of STEMI, either early or delayed coronary angiography is reasonable.[51] American Heart Association guidelines do not recommend emergency coronary angiography over delayed angiography in patients with ROSC in the absence of ST elevation, shock, electrical instability, signs of significant myocardial damage, or ongoing ischaemia.[54] PCI should only be performed in patients with culprit lesions on coronary angiography. Unnecessary PCI of stable lesions should be avoided in the early phase after OHCA, given the absence of benefit and increased risk of haemorrhagic complications and stent thrombosis in the setting of sudden cardiac arrest.[57][58]
Echocardiography can be used to assess cardiac contractility and check for structural abnormalities, valvular disorders and for evidence of tamponade. Left ventricular function should also be assessed 48 hours after ROSC, after the period of post-arrest myocardial stunning.[59] A normal imaging study in patients with ventricular arrhythmia suggests a primary electrical disorder.[7]
Exercise stress testing is useful for diagnosing and measuring response to treatment in patients with adrenergic-dependent rhythm disturbances, such as exercise-induced idiopathic monomorphic ventricular tachycardia (VT) or polymorphic VT. The 4-minute recovery QT interval after exercise testing can contribute to the diagnosis of long QT syndrome.[7][60]
Chest x-ray may reveal pneumothorax, pulmonary oedema, or other disorders of the lungs. The thoracic cage may show causes of, or complications from, cardiac arrest. Endotracheal tube placement should be evaluated if the patient is intubated.
A toxicology screen may be considered to rule out illicit substances that may predispose to ventricular arrhythmia.
Cardiac magnetic resonance imaging is used to identify ARVD or other primary cardiomyopathies. It is the preferred test to identify these disorders and should be done if other causes of sudden cardiac arrest are not discovered.
Coronary computed tomography angiography can be used to rule out coronary artery stenosis in patients who have a low probability of coronary artery disease.[7]
Signal-averaged ECG: may also be used to identify ARVD if other causes of sudden cardiac arrest are not discovered. Late potentials suggest the diagnosis.[7]
Electrophysiological studies: evaluation of primary arrhythmia or conduction abnormalities should be considered if no other cause of sudden cardiac arrest is found, or if there is potential for ablation of an arrhythmogenic source in patients with prior MI. Electrophysiological studies will delineate the arrhythmogenic focus.[7][61][62]
Screening of family members of patients with suspected or confirmed heritable syndromes, such as Brugada syndrome, long QT syndrome, and dilated or hypertrophic cardiomyopathy.[7]
Provocative tests can also be considered, such as sodium channel blocker testing for Brugada syndrome and adenosine testing to exclude latent pre-excitation.[7]
How to obtain an arterial blood sample from the radial artery.
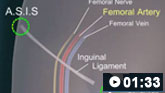
How to perform a femoral artery puncture to collect a sample of arterial blood.
Use of this content is subject to our disclaimer