The management of type 2 diabetes involves a range of behavioural and pharmacological interventions to prevent or delay complications and optimise quality of life.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Management needs to be individualised taking into account the particular requirements and circumstances of the patient, with glycaemic targets being discussed and agreed with them. Diet and lifestyle are central to this management. Structured education should be offered to the patient (and/or their family members or carers) at and around the time of diagnosis, with annual reinforcement and review.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Ongoing self-management education by a diabetes education nurse or dietitian promotes diabetes self-care and supports beneficial lifestyle changes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[103]Sherifali D, Bai JW, Kenny M, et al. Diabetes self-management programmes in older adults: a systematic review and meta-analysis. Diabet Med. 2015 Nov;32(11):1404-14.
https://onlinelibrary.wiley.com/doi/full/10.1111/dme.12780
http://www.ncbi.nlm.nih.gov/pubmed/25865179?tool=bestpractice.com
[104]Pillay J, Armstrong MJ, Butalia S, et al. Behavioral programs for type 2 diabetes mellitus: a systematic review and network meta-analysis. Ann Intern Med. 2015 Dec 1;163(11):848-60.
http://annals.org/aim/article/2446188/behavioral-programs-type-2-diabetes-mellitus-systematic-review-network-meta
http://www.ncbi.nlm.nih.gov/pubmed/26414227?tool=bestpractice.com
[105]Chatterjee S, Davies MJ, Heller S, et al. Diabetes structured self-management education programmes: a narrative review and current innovations. Lancet Diabetes Endocrinol. 2018 Feb;6(2):130-42.
http://www.ncbi.nlm.nih.gov/pubmed/28970034?tool=bestpractice.com
This requires general nutrition and health lifestyle knowledge and an individualised nutrition and exercise plan based on an initial assessment and treatment goals.
Interventions that enhance self-management can significantly reduce diabetes distress.[106]Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013 Sep;36(9):2551-8.
http://care.diabetesjournals.org/content/36/9/2551.long
http://www.ncbi.nlm.nih.gov/pubmed/23735726?tool=bestpractice.com
Care of adults with type 2 diabetes must include prompt management of all major cardiovascular risk factors to individualised targets. In addition to glucose control, this includes smoking cessation, blood pressure control, lipid control, consideration of antiplatelet use for patients with high cardiovascular disease (CVD) risk, and steps to reduce progression of diabetes-related kidney disease.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[107]Cheng J, Zhang W, Zhang X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014 May;174(5):773-85.
http://www.ncbi.nlm.nih.gov/pubmed/24687000?tool=bestpractice.com
The use of antihyperglycaemic agents is key to the management of type 2 diabetes. Drug selection is based on factors such as the patient’s clinical circumstances and preference; the drug’s effectiveness in terms of metabolic response and, importantly, cardiovascular and renal protection; as well as its safety, tolerability and monitoring requirements.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
In addition, special considerations should be given to those with type 2 diabetes and chronic kidney disease, such as using ACE inhibitors or angiotensin-II receptor antagonists and sodium-glucose cotransporter-2 (SGLT2) inhibitors when proteinuria is present.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Individualised care
Take an individualised approach to management that is tailored to the specific needs and circumstances of your patient. Take into account the patient’s:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
This is particularly important in the context of multimorbidity.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Discuss and agree an individual HbA1c target with the patient.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
To determine the most appropriate HbA1c target for your patient, check your local protocols and consult a specialist if needed. Consider relaxing the target HbA1c level on a case-by-case basis if appropriate for your individual patient, with particular consideration for those who are old or frail (see below).[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Encourage the patient to reach their target and maintain it, unless any resulting adverse effects (including hypoglycaemia) or the effort required to achieve their targets impairs their quality of life.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Use each review to reassess the patient’s needs and circumstances and consider whether to stop any medicines that are not effective.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Aim to make a routine assessment of frailty whenever you review an older person with diabetes.[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
[84]Sinclair A, Gallagher A. Managing frailty and associated comorbidities in older adults with diabetes: position statement on behalf of the Association of British Clinical Diabetologists (ABCD). 2019 [internet publication].
https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/ABCD-Position-Paper-Frailty.pdf
[85]Strain WD, Down S, Brown P, et al. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Ther. 2021 May;12(5):1227-47.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8099963
http://www.ncbi.nlm.nih.gov/pubmed/33830409?tool=bestpractice.com
Use a validated tool (e.g., the electronic Frailty Index [eFI], the Rockwood frailty score, or Timed Up and Go) to confirm clinical suspicion of frailty.[84]Sinclair A, Gallagher A. Managing frailty and associated comorbidities in older adults with diabetes: position statement on behalf of the Association of British Clinical Diabetologists (ABCD). 2019 [internet publication].
https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/ABCD-Position-Paper-Frailty.pdf
[85]Strain WD, Down S, Brown P, et al. Diabetes and frailty: an expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Ther. 2021 May;12(5):1227-47.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8099963
http://www.ncbi.nlm.nih.gov/pubmed/33830409?tool=bestpractice.com
Frail patients need a tailored approach to management; de-escalation of therapy is as important as intensification. Specifically bear in mind that:[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
Glycaemic targets recommended for good control in fit younger people are too tight for frail older patients.[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
[84]Sinclair A, Gallagher A. Managing frailty and associated comorbidities in older adults with diabetes: position statement on behalf of the Association of British Clinical Diabetologists (ABCD). 2019 [internet publication].
https://abcd.care/sites/abcd.care/files/site_uploads/Resources/Position-Papers/ABCD-Position-Paper-Frailty.pdf
Check your local protocols and consider consulting a specialist.
The most appropriate drug regimens for a frail patient, including choice of drug and optimal dose, will need careful consideration.[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
'Start low and go slow' when dosing and titrating medications in frail older adults.[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
Balance the intended benefit of treatment against the risk of adverse treatment effects.[108]Hambling CE, Khunti K, Cos X, et al. Factors influencing safe glucose-lowering in older adults with type 2 diabetes: a PeRsOn-centred ApproaCh To IndiVidualisEd (PROACTIVE) glycemic goals for older people: a position statement of Primary Care Diabetes Europe. Prim Care Diabetes. 2019 Aug;13(4):330-52.
https://www.primary-care-diabetes.com/article/S1751-9918(18)30300-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30792156?tool=bestpractice.com
In practice, unless directed otherwise by a specific clinical need or dosing regimen, allow 3-6 months to assess the impact of any intervention aimed at improving glycaemic control, whether pharmacological or non-pharmacological. Bear in mind that this timeframe will vary on an individual patient basis, and initial blood glucose levels; carefully consider how much of a risk the patient’s HbA1c might pose and step up to more intense interventions more quickly if needed.
Diet, physical activity, and sleep
Nutritional advice should be tailored to the needs of the individual patient, and be provided by a healthcare professional with specific expertise and competencies in nutrition.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The European Association for the Study of Diabetes (EASD) guidelines for the dietary management of diabetes note that a range of foods and dietary patterns are suitable for diabetes management.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
The National Institute for Health and Care Excellence (NICE) in the UK and the EASD recommend encouraging the patient to follow the same healthy eating advice as the general population.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
In particular, encourage the patient to:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Include minimally processed, high-fibre, low-glycaemic-index sources of carbohydrate in their diet, such as fruit, vegetables, nuts, seeds, wholegrains, and pulses
Eat low-fat dairy products and oily fish
Limit their intake of foods containing saturated and trans fatty acids
Minimise consumption of red and processed meats, sodium, sugar-sweetened beverages and refined grains.
Give individualised recommendations for carbohydrate and alcohol intake, and meal patterns.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Reducing the risk of hypoglycaemia should be a particular aim for a person using insulin or an insulin secretagogue.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Encourage an intake of free or added sugars below 10% of total energy intake.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Non-nutritive sweeteners can be used to replace sugars in foods and beverages.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Although limited substitution of sucrose-containing foods for other carbohydrates in the meal plan is allowable, advise the patient to take care to avoid excess energy intake.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Dietary fibre intake should be at least 35 g/day, with fibre-enriched foods and fibre supplements being considered when sufficient intake cannot be obtained from diet alone.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Discourage the patient from eating food marketed specifically for people with diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
There is ongoing debate about the potential role of low-carbohydrate diets in people with type 2 diabetes. Evidence suggests such diets can be safe and effective in the short term in managing weight and improving glycaemic control and cardiovascular risk.[109]Diabetes UK. Position statement: low carb diets for people with diabetes. May 2021 [internet publication].
https://www.diabetes.org.uk/professionals/position-statements-reports/food-nutrition-lifestyle/low-carb-diets-for-people-with-diabetes
One meta-analysis found no difference in glucose-lowering effects, weight, or low-density lipoprotein (LDL)-cholesterol levels between low- and high-carbohydrate diets at 1 year or later.[110]Snorgaard O, Poulsen GM, Andersen HK, et al. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2017 Feb 23;5(1):e000354.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5337734
http://www.ncbi.nlm.nih.gov/pubmed/28316796?tool=bestpractice.com
Another meta-analysis concluded there was moderate- to low-quality evidence that some patients can achieve remission of their type 2 diabetes by following a low-carb diet for 6 months.[111]Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021 Jan 13;372:m4743.
https://www.bmj.com/content/372/bmj.m4743.long
http://www.ncbi.nlm.nih.gov/pubmed/33441384?tool=bestpractice.com
The EASD does not recommend very-low-carbohydrate ketogenic diets.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
The American Diabetes Association (ADA) recommends considering reduction of the overall carbohydrate intake for adults to improve glycaemic control.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
A variety of dietary patterns, which encompass some of the key principles outlined above, are recommended by the EASD, including the Mediterranean, Nordic, and vegetarian/vegan diets.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Mediterranean or plant-based dietary patterns with high unsaturated fat content are also recommended by the European Society of Cardiology (ESC) to lower cardiovascular risk, with the former also recommended by the ADA.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
There is growing evidence that Mediterranean and vegetarian/vegan diets improve glycaemia and other cardiometabolic risk factors in people with type 2 diabetes, with the Nordic diet improving BMI and other cardiometabolic risk factors.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
[112]Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis. 2019 Jun;29(6):531-43.
https://www.primary-care-diabetes.com/article/S1751-9918(18)30300-0/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30952576?tool=bestpractice.com
[113]Pollakova D, Andreadi A, Pacifici F, et al. The impact of vegan diet in the prevention and treatment of type 2 diabetes: a systematic review. Nutrients. 2021 Jun 21;13(6):2123.
https://www.mdpi.com/2072-6643/13/6/2123
http://www.ncbi.nlm.nih.gov/pubmed/34205679?tool=bestpractice.com
[114]Tosatti JAG, Alves MT, Gomes KB. The role of the mediterranean dietary pattern on metabolic control of patients with diabetes mellitus: a narrative review. Adv Exp Med Biol. 2021;1307:115-28.
http://www.ncbi.nlm.nih.gov/pubmed/32253710?tool=bestpractice.com
However, care is required to avoid long-term exclusion of important nutrients.
There is insufficient evidence to recommend the routine use of herbal supplements and micronutrients (e.g. cinnamon, curcumin, vitamin D, aloe vera) to improve glycaemic control in patients with diabetes.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Integrate dietary advice with a personalised diabetes management plan that includes other aspects of lifestyle modification, including increasing physical activity and losing weight.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[115]National Institute for Health and Care Excellence. Preventing excess weight gain. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ng7
[116]National Institute for Health and Care Excellence. Weight management: lifestyle services for overweight or obese adults. May 2014 [internet publication].
https://www.nice.org.uk/guidance/ph53
[117]National Institute for Health and Care Excellence. Obesity: identification, assessment and management. July 2023 [internet publication].
https://www.nice.org.uk/guidance/cg189
[118]National Institute for Health and Care Excellence. Physical activity: brief advice for adults in primary care. May 2013 [internet publication].
https://www.nice.org.uk/guidance/ph44
In terms of physical activity, encourage the patient to:
Reduce sedentary behaviour and be more physically active.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[115]National Institute for Health and Care Excellence. Preventing excess weight gain. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ng7
Incorporate activity into their daily life (e.g., brisk walking, gardening, cycling).[115]National Institute for Health and Care Excellence. Preventing excess weight gain. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ng7
[117]National Institute for Health and Care Excellence. Obesity: identification, assessment and management. July 2023 [internet publication].
https://www.nice.org.uk/guidance/cg189
In this way, the patient can gradually increase the amount and intensity of activity they do.[115]National Institute for Health and Care Excellence. Preventing excess weight gain. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ng7
Do 45-60 minutes of moderate-intensity activity a day, particularly if they do not reduce their energy intake, in order to prevent obesity. Advise people who have been living with obesity and have lost weight that they may need to do 60-90 minutes of activity a day to avoid regaining weight.[117]National Institute for Health and Care Excellence. Obesity: identification, assessment and management. July 2023 [internet publication].
https://www.nice.org.uk/guidance/cg189
A systematic review and meta-analysis of observational studies concluded that physical activity, even below recommended amounts, was associated with reduced incidence of diabetes-related complications.[119]Rietz M, Lehr A, Mino E, et al. Physical activity and risk of major diabetes-related complications in individuals with diabetes: a systematic review and meta-analysis of observational studies. Diabetes Care. 2022 Dec 1;45(12):3101-11.
https://link.springer.com/chapter/10.1007/5584_2020_513
http://www.ncbi.nlm.nih.gov/pubmed/36455117?tool=bestpractice.com
Both the ADA and the ESC recommend advising patients about optimal minimum weekly levels of aerobic exercise: 150 minutes per week at moderate-intensity, or 75 minutes per week at vigorous-intensity (if there are no contraindications).[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
They further recommend that, in addition to aerobic exercise, resistance exercise should be performed at least twice weekly (provided it is not contraindicated) for additional benefit.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Both advise that all people with diabetes should be encouraged to increase their levels of any physical activity (e.g., walking, gardening, housework, yoga, dancing, swimming) above baseline, even if they do not meet optimal recommended exercise levels.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC additionally recommends the following regarding physical activity in those with type 2 diabetes:[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Exercise interventions should be adapted to any comorbidities and/or diabetes complications (e.g., frailty, neuropathy, retinopathy).
Consider behavioural theory-based interventions (e.g., goal-setting) to encourage exercise behaviours.
Use of structured exercise interventions for patients with established CVD to benefit metabolic control, exercise capacity, and quality of life, and reduce cardiovascular events. An exercise stress test should be considered in these patients prior to initiating a structured exercise programme.
If the patient has overweight:
NICE recommends an initial body weight loss target of 5% to 10%.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Evidence suggests that weight loss of >5% in adults with overweight or obesity improves glycaemic control, lipid levels, and blood pressure.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Weight loss management programmes with a healthy eating and physical activity plan resulting in an energy deficit have the potential for type 2 diabetes remission.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
[49]Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019 May;42(5):731-54.
https://care.diabetesjournals.org/content/42/5/731.long
http://www.ncbi.nlm.nih.gov/pubmed/31000505?tool=bestpractice.com
[120]Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018 Feb 10;391(10120):541-51.
http://www.ncbi.nlm.nih.gov/pubmed/29221645?tool=bestpractice.com
[121]Gregg EW, Chen H, Wagenknecht LE, et al; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012 Dec 19;308(23):2489-96.
https://jamanetwork.com/journals/jama/fullarticle/1486829
http://www.ncbi.nlm.nih.gov/pubmed/23288372?tool=bestpractice.com
The EASD recommends a low-energy total diet replacement programme (e.g. 3500 kJ/day [840 kcal/day] for 12-20 weeks), provided by trained health professionals, with carefully adjusted glucose-lowering and antihypertensive medications, to provide sufficient weight loss to induce remission of type 2 diabetes (10% to 15% body weight or greater).[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
The Diabetes Remission Clinical Trial (DiRECT) of supported weight loss management for people diagnosed with type 2 diabetes within the previous 6 years, and with a body mass index (BMI) of 27 kg/m² to 45 kg/m², found that almost half of participants achieved remission to a non-diabetic state and were off antidiabetic drugs at 12 months.[120]Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018 Feb 10;391(10120):541-51.
http://www.ncbi.nlm.nih.gov/pubmed/29221645?tool=bestpractice.com
At 2 years, more than one third of trial participants had sustained remission.[122]Lean ME, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019 May;7(5):344-55.
http://www.ncbi.nlm.nih.gov/pubmed/30852132?tool=bestpractice.com
Support the patient with evidence-based treatments to achieve and maintain weight loss. With the aid of trained health professionals, a variety of weight-loss diet types and macronutrient compositions can be used, provided that they meet other dietary recommendations.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
This includes nutritionally complete low-energy formula products, however, neither extreme high-carbohydrate, nor very-low-carbohydrate ketogenic diets are recommended by the EASD.[45]Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). Evidence-based European recommendations for the dietary management of diabetes. Diabetologia. 2023 Jun;66(6):965-85.
https://link.springer.com/article/10.1007/s00125-023-05894-8
http://www.ncbi.nlm.nih.gov/pubmed/37069434?tool=bestpractice.com
Following success of a pilot scheme, the National Health Service in England is expanding its programme of 3-month total diet replacement soups and shakes for people diagnosed with type 2 diabetes in the last 6 months.[123]NHS England. NHS to expand soups and shakes for people with type 2 diabetes. April 2023 [internet publication].
https://www.england.nhs.uk/2023/04/nhs-to-expand-soups-and-shakes-for-people-with-type-2-diabetes
Consider screening for sleep health, including symptoms of sleep disorders, disruption to sleep due to diabetes symptoms or management needs, and worry about sleep.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Consider referring to a sleep specialist.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Obesity and diabetes are risk factors for sleep apnoea, and inadequate sleep may affect glycaemic control.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Studies have shown that adherence to a healthy lifestyle (e.g., favourable diet, physical activity, non-smoking, moderate alcohol intake, and normal weight) is associated with a reduced relative risk of mortality in those with type 2 diabetes.[48]Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020 May;74(5):481-487.
http://www.ncbi.nlm.nih.gov/pubmed/32075860?tool=bestpractice.com
Cardiovascular risk management
Blood pressure (BP)
Consult your local protocols. Guidelines differ regarding recommended BP targets for those with type 2 diabetes.
In the most recent EUROASPIRE surveys (EAIV 2012/13 and EAV 2016/17), 80% of men and 87% of women with a diagnosis of diabetes also had a history of hypertension.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Optimal BP control reduces the risk of both microvascular and macrovascular complications.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
NICE recommends maintaining BP with lifestyle changes or drug treatment to targets of:[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
Below 135/85 mmHg for adults aged under 80 years, based on ambulatory or home BP monitoring (or <140/90 mmHg based on clinic BP)
Below 145/85 mmHg for patients aged 80 years and over, based on ambulatory or home BP monitoring (or <150/90 mmHg based on clinic BP)
Below 130/80 mmHg (clinic BP) for patients with chronic kidney disease (CKD) and albumin to creatinine ratio (ACR) of 70 mg/mmol or more; below 140/90 mmHg (clinic BP) for those with CKD and ACR <70 mg/mmol.
Although NICE recommends using clinical judgement for patients with frailty or multi-morbidity, their guideline committee concluded that there was no evidence to suggest BP targets should be different in people with type 2 diabetes; therefore, these recommendations for BP targets apply to people with and without type 2 diabetes.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
NICE also recommends using the same BP targets for people with and without cardiovascular disease.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
NICE highlights the importance of measuring standing as well as lying/sitting BP in people with type 2 diabetes, based on expert opinion that this group of patients is at higher than usual risk of postural hypotension:[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
If there is a drop in the systolic reading after standing for at least a minute (from lying or sitting) of 20 mmHg or more (or 10 mmHg or more in the diastolic reading), likely causes (including current medicines) should be considered and managed appropriately (e.g., falls prevention measures) and future BPs taken standing.
Further specialist evaluation should be considered if symptoms persist despite these measures, or if BP measurements (performed lying and standing) have not confirmed postural hypotension despite symptoms being suggestive.
If the patient has a significant postural drop in BP or symptoms of postural hypotension (e.g., falls, postural dizziness), NICE recommends that hypertension should be treated to a target based on standing measurements.
The 2023 ESC guideline on CVD management in diabetes recommends an individualised approach to treating hypertension in people with diabetes:[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
In people up to the age of 65: target systolic BP (SBP) to 130 mmHg, and <130 mmHg if tolerated (but no lower than 120 mmHg)
In older people (aged >65 years): target SBP to a range of 130 to 139 mmHg
SBP targeted to <130 mmHg may be considered to further reduce the risk of stroke in patients who have a particularly high risk of a cerebrovascular event.
Regardless of the specific BP goal, both lifestyle changes and antihypertensive medication may be needed to achieve BP control. Reduced sodium intake (to <100 mmol/day) and high intake of fruit, vegetables, and low-fat dairy products have all been shown to improve BP control.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
These lifestyle measures are supported by the ESC for people with type 2 diabetes and hypertension, in addition to alcohol restriction, increased exercise, and weight loss where appropriate.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
In the UK, NICE recommends a stepwise approach to pharmacological treatment of hypertension in people without CKD, or with CKD and an ACR ≤30 mg/mmol (ACR categories A1 and A2), as follows.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
For recommendations on choice of hypertensive agent in people with CKD and an ACR >30 mg/mmol (ACR category A3 or above), see below Specific pharmacotherapy considerations for patients with CKD.
Step 1[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
For initial treatment, give:
Step 2[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
If hypertension remains uncontrolled on first-line therapies:
Discuss, and support, adherence with antihypertensive medication
Step up to dual therapy by offering the choice of one of the following drugs in addition to step 1 treatment:
Step 3[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
Before considering next step treatment, discuss adherence with the patient and review their medications to ensure these are being taken at optimal tolerated doses.
If the patient’s BP remains uncontrolled despite step 2 therapies, offer a triple therapy combination of:
An ACE inhibitor or an angiotensin-II receptor antagonist, and
A calcium-channel blocker, and
A thiazide-like diuretic
Step 4[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
If BP is not controlled despite optimal tolerated doses of the triple-therapy medications in step 3, regard the patient as having resistant hypertension. Before considering further treatment, confirm elevated clinic BP measurements using ambulatory or home BP recordings, assess for postural hypotension, and discuss adherence. For people with confirmed resistant hypertension, consider adding a fourth antihypertensive drug or refer these patients to a hypertension specialist. Also consider possible secondary causes of hypertension (e.g., Conn’s adenoma, phaeochromocytoma, renovascular hypertension): in people with signs and symptoms suggesting a secondary cause, consider the need for specialist investigations and/or referral. Patients whose BP is still uncontrolled on four antihypertensive drugs should be referred to a specialist.
For people with cardiovascular disease, follow the recommendations for disease-specific indications in the NICE guideline for their condition.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
If their BP remains uncontrolled, offer antihypertensive drug treatment in line with the stepwise therapy outlined above.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
The ESC guideline takes a broadly similar approach to NICE but highlights additional key messages:[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
There is strong evidence to support the use of an ACE inhibitor or angiotensin-II receptor antagonist, particularly in patients who have microalbuminuria, proteinuria, or left ventricular hypertrophy. These drugs have renoprotective properties that go beyond their antihypertensive effects alone.
Dual therapy is recommended as first-line treatment because most patients will not achieve BP control on a single antihypertensive medication. These may be administered as a proprietary combination formulation (if available) to improve adherence and achieve quicker BP control. A combination of an ACE inhibitor or angiotensin-II receptor antagonist, with a calcium-channel blocker or a thiazide-like diuretic is recommended.
Beta-blockers should be considered at any step in hypertension treatment if there is a specific indication (e.g., coronary artery disease, atrial fibrillation, heart failure).
There is an increasing drive to incorporate the use of home BP monitoring into the diagnosis and management of hypertension in adults, including those with diabetes.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[124]Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013 Jul 3;310(1):46-56.
https://jamanetwork.com/journals/jama/fullarticle/1707720
http://www.ncbi.nlm.nih.gov/pubmed/23821088?tool=bestpractice.com
Lipids
Consult your local protocols. Guidelines recommend differing approaches to lipid-modification therapy.
NICE recommends:
Using a validated risk assessment tool such as QRISK3 to assess CVD risk within the next 10 years in people with type 2 diabetes aged between 25 and 84 years (those aged 85 years and over should be considered at increased CVD risk due to age alone, especially if they smoke and/or have elevated BP).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
QRISK®3 calculator
Opens in new window
In its guideline on type 2 diabetes (last updated in 2022), NICE recommends to use QRISK2 to assess cardiovascular disease risk in adults with type 2 diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
However, NICE’s 2023 guideline on CVD recommends using QRISK3.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE acknowledges that it may be necessary to use QRISK2 until electronic clinical systems are updated with QRISK3.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Depending on local availability, either QRISK2 or QRISK3 is a reasonable tool for assessing CVD risk in adults with type 2 diabetes.
NICE does specify certain populations for whom QRISK3 should be used (as QRISK2 may underestimate CVD risk in these patients), including people with severe mental illness, systemic lupus erythematosus, migraine, or erectile dysfunction, and people taking corticosteroids or atypical antipsychotics.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE also notes that CVD risk tools (including QRISK3) may underestimate risk in some groups (e.g., those treated for HIV, those who have recently stopped smoking, etc.) and emphasise that clinical judgement is needed to interpret risk scores.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE does not recommend use of a risk assessment tool for people at high risk of CVD, including those with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m² and/or albuminuria, or an inherited disorder of lipid metabolism (e.g., familial hypercholesterolaemia).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Offering high-intensity statin therapy (i.e., atorvastatin at the low end of the high-intensity dose range) for the primary prevention of CVD for people with (and without) type 2 diabetes who have ≥10% 10-year risk (estimated using QRISK3) of developing CVD.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE notes not to rule out treatment with atorvastatin for primary prevention just because the patient’s 10-year risk score is less than 10% if they have an informed preference for taking a statin or there is concern the risk may be underestimated.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE also recommends considering this treatment for those aged 85 years and older, with an awareness for the factors that may make treatment inappropriate (e.g., life expectancy, frailty, comorbidities, or other current medicines).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Offering high-intensity statin therapy (i.e., atorvastatin at the high end of the high-intensity dose range) for secondary prevention for people with (and without) diabetes with established CVD, irrespective of their cholesterol level and provided they have normal renal function.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
A lower dose should be offered for secondary prevention if the person is at high risk of adverse effects from statins or is taking other drugs that could interact with the statin, or if they have a preference for a lower dose.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
People with CKD who require secondary prevention of CVD should be offered atorvastatin at the low end of the high-intensity dose range (i.e., the same as for primary prevention).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
The decision to start (or escalate) lipid-lowering therapy should be made with the patient following an informed discussion about the risks and benefits of treatment, with consideration of individual patient factors (e.g., preferences, comorbidities, other medicines).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Statins are contraindicated in pregnancy. Statins should be stopped 3 months before the patient attempts to conceive and should not be restarted until breastfeeding is finished.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Before starting lipid-modification therapy, a clinical assessment should be carried out, including review of smoking and diabetes status, alcohol consumption, BP, and BMI (or other measure of obesity).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Baseline blood tests should be performed, including renal function, a liver transaminase level, and full lipid profile (i.e., total cholesterol and high-density lipoprotein [HDL]-cholesterol and triglyceride levels, from which non-HDL and low-density lipoprotein [LDL]-cholesterol are calculated; a fasting sample is not needed).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Blood tests for thyroid-stimulating hormone (TSH) level should be requested if there are symptoms of thyroid dysfunction, and creatinine kinase levels should be performed if the person has had persistent generalised unexplained muscle symptoms (pain, tenderness, or weakness).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Clinical findings, full lipid profile, and family history should be used to assess likelihood of a familial lipid disorder (instead of basing the decision on lipid values alone).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Familial lipid disorders are outside the scope of this topic: for more information about when to suspect and how to diagnose familial hypercholesterolaemia, see Hypercholesterolaemia.
Arrange for specialist assessment of people with a total cholesterol concentration of more than 9.0 mmol/L or a non-HDL-cholesterol concentration of more than 7.5 mmol/L even in the absence of a first-degree family history of premature coronary heart disease.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Comorbidities and secondary causes of dyslipidaemia should be treated (and if appropriate, lifestyle changes discussed) at the same time as initiation of statin therapy for secondary prevention (or before starting a statin for primary prevention).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Consistent data have demonstrated the efficacy of statins in preventing cardiovascular events and reducing cardiovascular mortality in patients with diabetes, with no evidence for sex differences; their use is associated with a limited number of adverse events.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
In terms of monitoring, NICE recommends the following:[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
At 2-3 months after initiating or changing a lipid-lowering treatment, perform a full lipid profile and liver transaminase level (liver transaminase should be checked again at 12 months; no further repeat liver transaminase tests are then necessary unless clinically indicated).
In those on a statin for primary prevention, aim for a greater than 40% reduction in non-HDL-cholesterol, and in those using a statin for secondary prevention, aim for LDL-cholesterol of 2.0 mmol/L or less (or non-HDL-cholesterol of 2.6 mmol/L or less). If the appropriate lipid target is not achieved:
Discuss adherence and timing of dose
Optimise adherence to diet and lifestyle measures
Consider increasing the statin intensity/dose if appropriate. If the person has CKD and eGFR is 30 mL/min/1.73 m² or more, the dose of atorvastatin should be increased (if eGFR is <30 mL/min/1.73 m², any dose increase should be agreed with a renal specialist).
If adverse effects are reported with high-intensity statin therapy, consider the following options with the patient:
Stop the statin and reintroduce it once symptoms resolve to check if they are statin-related; or
Change to an alternative high-intensity statin; or
Reduce the dose; or
Change to a lower-intensity statin.
Review medication annually. Use these reviews to discuss medication adherence and lifestyle modification and to address CVD risk factors. Those on secondary prevention should be offered an annual full lipid profile to inform the discussion (and this should also be considered for those on primary prevention).
Do not stop statins because of an increase in blood glucose level or HbA1c.
ESC guidelines recommend screening patients with diabetes for severe target organ damage (TOD) and assessing for symptoms and medical history suggestive of atherosclerotic cardiovascular disease (ASCVD) to understand their cardiovascular risk.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Where neither symptomatic ASCVD nor severe TOD are present, they recommend use of the SCORE2-Diabetes tool to estimate 10-year CVD risk.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC categorises patients with type 2 diabetes according to these assessments into the following cardiovascular risk groups (used to guide management):[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Very high risk
High risk
Moderate risk
Based on these categories, the ESC recommends for patients with type 2 diabetes who are:[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
At low cardiovascular risk: no clear target recommendations can be made due to lack of evidence
At moderate cardiovascular risk: an LDL-cholesterol target of <2.6 mmol/L (<100 mg/dL)
At high cardiovascular risk: an LDL-cholesterol target of <1.8 mmol/L (<70 mg/dL) and LDL-cholesterol reduction of at least 50%; a secondary goal of a non-HDL-cholesterol target of <2.6 mmol/L (<100 mg/dL)
At very high cardiovascular risk: an LDL-cholesterol target of <1.4 mmol/L (<55 mg/dL) and LDL-cholesterol reduction of at least 50%; a secondary goal of a non-HDL-cholesterol target of <2.2 mmol/L (<85 mg/dL).
The ESC recommends statins as first-line lipid-lowering treatment in patients with diabetes and high LDL-cholesterol levels.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The cardiovascular risk profile (very high, high, moderate) of the individual patient and the corresponding LDL-cholesterol (or non-HDL-cholesterol) target levels should be used to determine statin administration.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC recommends intensive LDL-cholesterol lowering with statins for those with CKD.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Specific regimens for other lipid-lowering therapies are outside the scope of this topic: refer to Hypercholesterolaemia for more information. The following is general guidance.
Both NICE and ESC guidelines recommend that if the appropriate lipid target in a person with established ASCVD is not reached on maximum tolerated statin therapy, intensification of lipid-lowering therapy (i.e., additional lipid-lowering therapies) should be considered on an individual basis in collaboration with the patient.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
For some patients with diabetes and established CVD who have persistently elevated LDL-cholesterol despite maximally tolerated statin therapy, addition of ezetimibe or a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor monoclonal antibody may confer clinical benefit.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[126]Giugliano RP, Cannon CP, Blazing MA, et al; IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2018 Apr 10;137(15):1571-82.
http://www.ncbi.nlm.nih.gov/pubmed/29263150?tool=bestpractice.com
[127]Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017 May 4;376(18):1713-22.
https://www.nejm.org/doi/10.1056/NEJMoa1615664
http://www.ncbi.nlm.nih.gov/pubmed/28304224?tool=bestpractice.com
[128]Squizzato A, Suter MB, Nerone M, et al. PCSK9 inhibitors for treating dyslipidemia in patients at different cardiovascular risk: a systematic review and a meta-analysis. Intern Emerg Med. 2017 Oct;12(7):1043-53.
http://www.ncbi.nlm.nih.gov/pubmed/28695455?tool=bestpractice.com
[129]Lee YJ, Cho JY, You SC, et al. Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial. Eur Heart J. 2023 Mar 14;44(11):972-83.
https://academic.oup.com/eurheartj/article/44/11/972/6931821?login=false
http://www.ncbi.nlm.nih.gov/pubmed/36529993?tool=bestpractice.com
The BMJ: PCSK9 inhibitors and ezetimibe for the reduction of cardiovascular events: a clinical practice guideline with risk-stratified recommendations
Opens in new window
Further, NICE recommends considering the addition of ezetimibe (to maximum tolerated statin therapy) in patients with established CVD to further reduce their CVD risk even if the lipid target for secondary prevention is met with a statin alone.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
The ESC recommends a statin plus ezetimibe combination therapy for patients not meeting their target LDL-cholesterol on statin therapy alone (there is no specification of cardiovascular risk category given for this recommendation), or as an option for intensive LDL-cholesterol lowering in patients with diabetes and CKD.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Other lipid-lowering therapies should also be considered on an individual basis if statin therapy is contraindicated, or is not tolerated at any intensity/dose, and may be required in addition to statin therapy if the patient has additional indications for lipid-lowering treatment (e.g., hypertriglyceridaemia, a familial lipid disorder).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Smoking cessation
If the patient smokes, give advice on smoking cessation and information on accessing smoking cessation services.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[130]National Institute for Health and Care Excellence. Tobacco: preventing uptake, promoting quitting and treating dependence. January 2023 [internet publication].
https://www.nice.org.uk/guidance/ng209
The ESC recommends pharmacological therapies (e.g., nicotine replacement therapy, varenicline, bupropion) should be considered, as well as counselling, to improve cessation success rate.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Antiplatelet therapy
People with type 2 diabetes and CVD will need antiplatelet therapy for secondary prevention. This will usually be in the form of low-dose aspirin or clopidogrel.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Antiplatelet therapy has been found to reduce the risk of stroke, myocardial infarction, or vascular death.[131]Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002 Jan 12;324(7329):71-86.
http://www.bmj.com/content/324/7329/71.full
http://www.ncbi.nlm.nih.gov/pubmed/11786451?tool=bestpractice.com
The role of antiplatelets in primary prevention of CVD is unclear and guidelines differ in their recommendations. Consult your local protocols.
NICE recommends against routine antiplatelet therapy (aspirin or clopidogrel) as primary prevention for adults with type 2 diabetes without CVD.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
This is because the increased risk of major bleeding is considered to outweigh any potential benefits from primary prevention.[132]Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep 7;42(34):3227-337.
https://academic.oup.com/eurheartj/article/42/34/3227/6358713
http://www.ncbi.nlm.nih.gov/pubmed/34458905?tool=bestpractice.com
The ASCEND trial compared low-dose aspirin with placebo among 15,480 adults with diabetes but no evident CVD. It found that the benefits of aspirin use in preventing serious vascular events was largely counterbalanced by the increased risk of major bleeding events over a mean follow-up of 7.4 years.[133]ASCEND Study Collaborative Group; Bowman L, Mafham M, Wallendszus K, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018 Oct 18;379(16):1529-39.
https://www.nejm.org/doi/10.1056/NEJMoa1804988
http://www.ncbi.nlm.nih.gov/pubmed/30146931?tool=bestpractice.com
However, the ESC recommends that low-dose aspirin can be considered for prevention of the first severe vascular event in adults with type 2 diabetes without a history of symptomatic ASCVD or revascularisation, provided there are no clear contraindications.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
They acknowledge that therapy should be individualised, as those with a higher cardiovascular risk may benefit more from antiplatelet therapy.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
They recommend the use of low-dose aspirin in patients with CKD and ASCVD.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC also recommends that concomitant use of an appropriate proton-pump inhibitor should be considered (based on the individual bleeding risk of the patient) for patients taking a single antiplatelet drug (e.g., low-dose aspirin) to reduce the gastrointestinal bleeding risk highlighted by the ASCEND trial (in which three-quarters of patients were not taking a proton-pump inhibitor).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
They make the same recommendation for those taking a single anticoagulant.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Further, they recommend that an appropriate proton-pump inhibitor is used when a combination of antithrombotic drugs is being taken.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Specific pharmacotherapy considerations for patients with CKD
If your patient with type 2 diabetes has chronic kidney disease (CKD) or develops it at any point post-diagnosis, NICE recommends to:
Give an angiotensin-II receptor antagonist or an ACE inhibitor (titrated to the highest licensed dose that the person can tolerate) if albumin-to-creatinine ratio (ACR) is 3 mg/mmol or more (i.e., clinically important proteinuria).[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
Give an SGLT2 inhibitor (in addition to the angiotensin-II receptor antagonist or ACE inhibitor) if the patient is already taking an angiotensin-II receptor antagonist or an ACE inhibitor (titrated to the highest licensed dose that they can tolerate) and:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[Evidence A]61a0123d-3704-47cf-8cd7-ef9b0668234eguidelineAWhat is the clinical effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors as add-on pharmacotherapy for adults with chronic kidney disease (CKD) and type 2 diabetes?[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Consider an SGLT2 inhibitor (in addition to the angiotensin-II receptor antagonist or ACE inhibitor) if the patient is already taking an angiotensin-II receptor antagonist or an ACE inhibitor (titrated to the highest licensed dose that they can tolerate) and:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[Evidence A]61a0123d-3704-47cf-8cd7-ef9b0668234eguidelineAWhat is the clinical effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors as add-on pharmacotherapy for adults with chronic kidney disease (CKD) and type 2 diabetes?[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient has stage 3 or stage 4 CKD (i.e., with an eGFR <60 mL/min/1.73 m²) associated with type 2 diabetes with albuminuria, consider finerenone (a non-steroidal mineralocorticoid receptor antagonist) if:[134]National Institute for Health and Care Excellence. Finerenone for treating chronic kidney disease in type 2 diabetes. March 2023 [internet publication].
https://www.nice.org.uk/guidance/ta877
It is used as an add-on to optimised standard care (which should include, unless they are unsuitable, the highest tolerated licensed doses of ACE inhibitors or angiotensin-II receptor antagonists and SGLT2 inhibitors) and
The patient has an eGFR of ≥25 mL/min/1.73 m².
If the patient has CKD and requires an antihypertensive agent:
Follow the recommendations outlined in Cardiovascular risk management, which also apply to people without CKD, if the patient has an ACR ≤30 mg/mmol (ACR categories A1 and A2).[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
Give an angiotensin-II receptor antagonist or an ACE inhibitor (titrated to the highest licensed dose that the person can tolerate) if the patient has an ACR >30 mg/mmol (ACR category A3 or above).[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
Where possible, treat hypertension in people with CKD with drugs taken only once daily.[82]National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Nov 2023 [internet publication].
https://www.nice.org.uk/guidance/ng136
These drugs should be added to optimised standard care (see Antihyperglycaemic pharmacotherapy section).
SGLT2 inhibitors are not suitable for everyone and should only be used within their marketing authorisation; some SGLT2 inhibitors are not licensed for this indication in some regions, including the UK.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
These recommendations from NICE are based on evidence from randomised controlled trials which showed that:
SGLT2 inhibitors reduce the risk of CKD progression, mortality and cardiovascular events in adults with type 2 diabetes and CKD.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[Evidence A]61a0123d-3704-47cf-8cd7-ef9b0668234eguidelineAWhat is the clinical effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors as add-on pharmacotherapy for adults with chronic kidney disease (CKD) and type 2 diabetes?[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Angiotensin-II receptor antagonists reduce the risk of end-stage renal disease and heart failure.[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
There is no clear difference between ACE inhibitors and angiotensin-II receptor antagonists on a number of outcomes, including end-stage renal disease, all-cause mortality, cardiovascular mortality, and hospitalisation.[86]National Institute for Health and Care Excellence. Chronic kidney disease: assessment and management. November 2021 [internet publication].
https://www.nice.org.uk/guidance/ng203
Pharmacotherapy recommendations for patients with type 2 diabetes and CKD provided by the Kidney Disease: Improving Global Outcomes (KDIGO) 2022 clinical practice guideline are largely aligned with the NICE guidelines outlined above.[135]Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int. 2022 Nov;102(5s):S1-127.
https://www.kidney-international.org/article/S0085-2538(22)00507-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36272764?tool=bestpractice.com
They also suggest that a non-steroidal mineralocorticoid receptor antagonist with proven kidney or cardiovascular benefit be considered for patients with type 2 diabetes, eGFR ≥25 mL/min/1.73 m², normal serum potassium concentration, and clinically important proteinuria, despite maximum tolerated dose of an angiotensin-II receptor antagonist or an ACE inhibitor.[135]Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int. 2022 Nov;102(5s):S1-127.
https://www.kidney-international.org/article/S0085-2538(22)00507-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36272764?tool=bestpractice.com
The United Kingdom Kidney Association (UKKA) in 2023 recommends use of an SGLT2 inhibitor in people with type 2 diabetes and CKD (irrespective of primary kidney disease, but excluding those with polycystic kidney disease or kidney transplant recipients) who have:[136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
An eGFR of 20-45 mL/min/1.73 m², or
An eGFR of >45 mL/min/1.73 m² and a urinary albumin-to-creatinine ratio (uACR) of ≥25 mg/mmol (urinary protein-to-creatinine ratio of 35 mg/mmol can be considered equivalent), or
Symptomatic heart failure, irrespective of ejection fraction, or
Established coronary disease.
The UKKA also suggests that, in people with type 2 diabetes and CKD (excluding kidney transplant recipients), an SGLT2 inhibitor should be:[136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
Initiated if eGFR is >45-60 mL/min/1.73 m² and uACR is <25 mg/mmol to modify cardiovascular risk and slow rate of kidney function decline (recognising that effects on glycaemic control will be limited).
Considered if eGFR is <20 mL/min/1.73 m² to slow kidney disease progression, based on evidence that this benefit is still seen at low eGFR.
The UKKA recommends that SGLT2 inhibitors are continued until renal replacement therapy is required.[136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
The 2023 ESC guidelines on CVD management in diabetes recommend treating patients with type 2 diabetes and CKD with an SGLT2 inhibitor and/or finerenone, since these reduce cardiovascular risk and kidney failure risk on top of standard care (e.g., ACE inhibitor or angiotensin-II receptor antagonist).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Specifically, in addition to use of an ACE inhibitor or angiotensin-II receptor antagonist, they recommend use of:[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC also makes recommendations in a 2023 update to their heart failure guidelines for SGLT2 inhibitors and finerenone in patients with type 2 diabetes and CKD to reduce the risk of heart failure hospitalisation (both SGLT2 inhibitors and finerenone) or cardiovascular death (SGLT2 inhibitors).[137]McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622666?tool=bestpractice.com
Blood glucose targets and self-monitoring
HbA1c goals should be individualised at all stages of management; the patient should be involved in any decisions about their individual glycaemic target.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[138]Laiteerapong N, Cooper JM, Skandari MR, et al. Individualized glycemic control for US adults with type 2 diabetes: a cost-effectiveness analysis. Ann Intern Med. 2018 Feb 6;168(3):170-8.
http://www.ncbi.nlm.nih.gov/pubmed/29230472?tool=bestpractice.com
[139]Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011 Apr 19;154(8):554-9.
http://www.ncbi.nlm.nih.gov/pubmed/21502652?tool=bestpractice.com
Individualised HbA1c goals improve quality of life compared with uniform tight control.[139]Ismail-Beigi F, Moghissi E, Tiktin M, et al. Individualizing glycemic targets in type 2 diabetes mellitus: implications of recent clinical trials. Ann Intern Med. 2011 Apr 19;154(8):554-9.
http://www.ncbi.nlm.nih.gov/pubmed/21502652?tool=bestpractice.com
In the UK, NICE recommends:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient manages their type 2 diabetes either with lifestyle and diet, or with lifestyle and diet combined with a single drug that is not associated with hypoglycaemia, support them to aim for an HbA1c level of 48 mmol/mol (6.5%)
If the patient is on a drug associated with hypoglycaemia, support them to aim for an HbA1c level of 53 mmol/mol (7.0%).
The ESC recommends tight glycaemic control (HbA1c <7.0%; if appropriate/able) to reduce microvascular complications, and they advise to consider the same for reducing coronary artery disease in the long-term (preferably using agents with proven cardiovascular benefit).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Consider a slightly higher HbA1c level on a case-by-case basis for:[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Patients who are older or frail
Patients who are unlikely to achieve longer-term risk-reduction benefits (e.g., those with a reduced life expectancy)
Patients for whom tight blood glucose control poses a high risk of the consequences of hypoglycaemia, including people who are at risk of falling; people who have impaired awareness of hypoglycaemia; those who drive or operate machinery as part of their job
Patients for whom intensive management is not appropriate (e.g., people with significant comorbidities).
Bear in mind that the most appropriate drug regimens for a frail patient, including choice of drug and optimal dose, will need careful consideration and are likely to differ from those recommended for younger, fit patients.[83]Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty – a national collaborative stakeholder initiative. Diabet Med. 2018 Jul;35(7):838-45.
https://onlinelibrary.wiley.com/doi/10.1111/dme.13644
http://www.ncbi.nlm.nih.gov/pubmed/29633351?tool=bestpractice.com
The ADA recommends use of medicines with low hypoglycaemia risk for older adults, and deintensification and/or simplification of treatment plans (within individualised glycaemic goals) in this population when the harms or burdens of treatment may outweigh the benefits.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
If the patient drives, ensure they are aware of the relevant local advice on plasma glucose level. In the UK, the Driver and Vehicle Licensing Agency advises to aim for a level of at least 5 mmol/L (90 mg/dL) before driving.[140]Driver and Vehicle Licensing Agency. Information for drivers with diabetes. June 2021 [internet publication].
https://www.gov.uk/government/publications/information-for-drivers-with-diabetes
Self-monitoring of glucose levels may help with self-management and medication adjustment, particularly in people taking insulin. It can give insight into the impact of lifestyle and medication management on blood glucose and symptoms, particularly when combined with education and support; glucose monitoring plans should be individualised.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Traditionally, self-monitoring involves glucose levels being measured from capillary blood samples (self-monitoring of blood glucose, SMBG) that is using finger-stick blood glucose testing.
NICE and the European Association for the Study of Diabetes/American Diabetes Association (EASD/ADA) recommend SMBG as an option for people with type 2 diabetes who are using insulin.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
NICE also recommends routinely offering SMBG for adults with type 2 diabetes if:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
There is evidence of hypoglycaemic episodes, or
The patient is on oral medication that may increase their risk of hypoglycaemia while driving or operating machinery, or
The patient is pregnant, or is planning to become pregnant.
NICE recommends considering short-term SMBG in adults with type 2 diabetes (and reviewing treatment as necessary):[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
When starting treatment with oral or intravenous corticosteroids, or
To confirm suspected hypoglycaemia.
The newer technology of continuous glucose monitoring (CGM) is now also being used. CGM involves a small, disposable device with a subcutaneous sensor constantly attached to the skin, which measures glucose levels in interstitial fluid, and sends the readings to a display device or smart device.[141]Lewis DM, Oser TK, Wheeler BJ. Continuous glucose monitoring. BMJ. 2023 Mar 3;380:e072420.
http://www.ncbi.nlm.nih.gov/pubmed/36868576?tool=bestpractice.com
The CGM devices may provide real-time data (rtCGM) or intermittently scanned data (isCGM). CGM can provide more information than SMBG, but it might not be readily available in all regions for people with type 2 diabetes.
NICE recommends offering CGM to adults with type 2 diabetes if they are:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The EASD/ADA also state that CGM provides more information, and has clear advantages over SMBG and may be useful for people with type 2 diabetes, particularly those treated with insulin.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
CGM use is recommended by the ADA for people with diabetes that are at high risk of hypoglycaemia.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
NICE notes that CGM should be provided by a team with expertise in its use, and appropriate patient support and education provided.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Capillary blood glucose measurements will still need to be carried out to check the accuracy of the CGM device, and need to be available as a back-up should the CGM device fail.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Antihyperglycaemic pharmacotherapy: overarching principles
If the patient is unable to meet their individualised HbA1c goal with lifestyle interventions, pharmacotherapy is recommended to reduce risk of both microvascular (nephropathy, retinopathy, neuropathy) and macrovascular (coronary artery, cerebrovascular, and peripheral vascular disease) complications.[142]UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998 Sep 12;352(9131):854-65.
http://www.ncbi.nlm.nih.gov/pubmed/9742977?tool=bestpractice.com
[143]Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89.
https://www.nejm.org/doi/full/10.1056/NEJMoa0806470
http://www.ncbi.nlm.nih.gov/pubmed/18784090?tool=bestpractice.com
In general, global pharmacotherapy strategies have shifted to focus on the improvement of cardiovascular and kidney disease outcomes instead of being solely led by glycaemic control. This is based on evidence from high-quality randomised trials demonstrating the benefits of some agents on atherosclerotic cardiovascular disease (CVD), heart failure, and chronic kidney disease (CKD), largely independent of their glucose-lowering potential.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
In particular, SGLT2 inhibitors appear to have benefits in patients with type 2 diabetes who have multiple risk factors for CVD, and both SGLT2 inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists appear beneficial in those with established CVD and/or renal disease.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[144]Patel A, MacMahon S, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 6;358(24):2560-72.
https://www.nejm.org/doi/full/10.1056/NEJMoa0802987
http://www.ncbi.nlm.nih.gov/pubmed/18539916?tool=bestpractice.com
[145]Gerstein HC, Miller ME, Byington RP, et al; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545-59.
https://www.nejm.org/doi/full/10.1056/NEJMoa0802743
http://www.ncbi.nlm.nih.gov/pubmed/18539917?tool=bestpractice.com
[146]Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021 May 11;373:n1091.
https://www.bmj.com/content/373/bmj.n1091.long
http://www.ncbi.nlm.nih.gov/pubmed/33975892?tool=bestpractice.com
In addition, metformin has likely cardiovascular benefits.[147]Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017 Sep;60(9):1620-9.
https://link.springer.com/article/10.1007%2Fs00125-017-4337-9
http://www.ncbi.nlm.nih.gov/pubmed/28770324?tool=bestpractice.com
In line with global trends, NICE now recommends an approach to managing type 2 diabetes that moves away from one that focuses solely on achieving individualised HbA1c targets, to one which incorporates therapies that not only reduce blood glucose levels, but have proven cardiovascular and renal benefits.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The recommendations in this topic are based primarily on the NICE approach.
It should be noted that in older studies, such as ACCORD, ADVANCE, and the Veterans Affairs Diabetes Trial, use of multiple drugs to achieve near-normal HbA1c was either not beneficial or increased mortality in type 2 diabetes patients with CVD or high CVD risk.[144]Patel A, MacMahon S, et al; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008 Jun 6;358(24):2560-72.
https://www.nejm.org/doi/full/10.1056/NEJMoa0802987
http://www.ncbi.nlm.nih.gov/pubmed/18539916?tool=bestpractice.com
[145]Gerstein HC, Miller ME, Byington RP, et al; Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545-59.
https://www.nejm.org/doi/full/10.1056/NEJMoa0802743
http://www.ncbi.nlm.nih.gov/pubmed/18539917?tool=bestpractice.com
[148]Gerstein HC, Miller ME, Genuth S, et al; ACCORD Study Group. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011 Mar 3;364(9):818-28.
http://www.ncbi.nlm.nih.gov/pubmed/21366473?tool=bestpractice.com
[149]Reaven PD, Emanuele NV, Wiitala WL, et al; VADT Investigators. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. N Engl J Med. 2019 Jun 6;380(23):2215-24.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6706253
http://www.ncbi.nlm.nih.gov/pubmed/31167051?tool=bestpractice.com
[150]Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129-39.
https://www.nejm.org/doi/full/10.1056/NEJMoa0808431
http://www.ncbi.nlm.nih.gov/pubmed/19092145?tool=bestpractice.com
However, SGLT2 inhibitors were not available and GLP-1 receptor agonists were infrequently used in those studies.
Choice of agents should be individualised, taking into account patient preferences, needs and clinical circumstances (e.g. weight, comorbidities, risks of complications), the effectiveness of the agent in terms of metabolic response and cardiovascular and renal protection, adverse effects, and other factors.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
When choosing, reviewing and changing medicines, always:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If combination therapy is indicated, introduce drugs in a stepwise manner, checking for tolerability and effectiveness of each drug.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If an adult with type 2 diabetes is symptomatically hyperglycaemic at any phase of treatment, consider rescue therapy with insulin or a sulfonylurea, and review treatment when blood glucose control has been achieved.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Antihyperglycaemic pharmacotherapy: initial treatment
NICE has produced a flowchart summarising its recommendations for choosing first-line medicines in adults with type 2 diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
NICE: Type 2 diabetes in adults: choosing medicines
Opens in new window
[Figure caption and citation for the preceding image starts]: Type 2 diabetes in adults: choosing medicines - how to choose first-line medicinesNICE NG28 (2022) Type 2 diabetes in adults: choosing medicines; used with permission [Citation ends].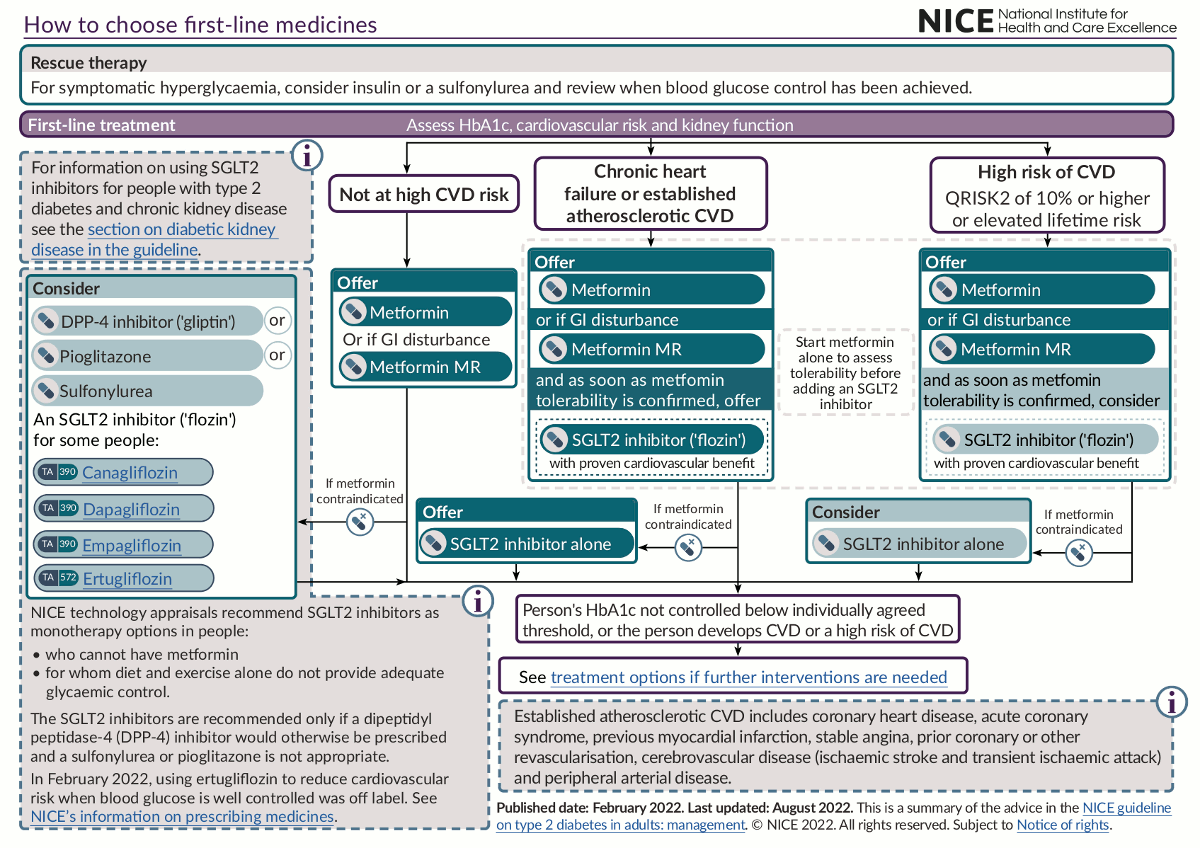
Assess the patient's HbA1c, cardiovascular risk, and kidney function.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Determine whether the patient has chronic heart failure or established atherosclerotic cardiovascular disease (CVD; includes coronary heart disease, acute coronary syndrome, previous myocardial infarction, stable angina, prior coronary or other revascularisation, cerebrovascular disease [ischaemic stroke and transient ischaemic attack] and peripheral arterial disease), or is at high risk of developing cardiovascular disease. Use this cardiovascular risk assessment to tailor drug treatment.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
In its guideline on type 2 diabetes (last updated in 2022), NICE recommends to use QRISK2 to assess cardiovascular disease risk in adults with type 2 diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
However, NICE’s 2023 guideline on CVD recommends using QRISK3. NICE acknowledges that it may be necessary to use QRISK2 until electronic clinical systems are updated with QRISK3.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
We refer to QRISK3 below but, depending on local availability, either QRISK2 or QRISK3 is a reasonable tool for assessing CVD risk in adults with type 2 diabetes.
Note that NICE does specify certain populations (e.g., people with severe mental illness) for whom QRISK3 should be used, as QRISK2 may underestimate CVD risk in these patients.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Further, NICE notes that any CVD risk tool (including QRISK3) may underestimate risk in some groups (e.g., those treated for HIV) and they emphasise using clinical judgement to interpret risk scores.[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
NICE does not recommend use of CVD risk tools for people at high risk of CVD (e.g., those with eGFR <60 mL/min/1.73 m² and/or albuminuria).[125]National Institute for Health and Care Excellence. Cardiovascular disease: risk assessment and reduction, including lipid modification. Dec 2023 [internet publication].
https://www.nice.org.uk/guidance/ng238/chapter/Recommendations
Metformin
Give metformin as first-line drug treatment to all adults with type 2 diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The dose of immediate-release metformin should be gradually increased over several weeks to minimise the risk of gastrointestinal adverse effects.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If gastrointestinal disturbance occurs with immediate-release metformin, a modified-release formulation can be used instead.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
However, modified-release metformin is associated with statistically worse but likely clinically similar HbA1c lowering and minimal improvement of gastrointestinal intolerance compared to immediate-release metformin.[151]Abrilla AA, Pajes ANNI, Jimeno CA. Metformin extended-release versus metformin immediate-release for adults with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2021 Aug;178:108824.
https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(21)00183-2/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33887354?tool=bestpractice.com
If metformin is contraindicated or not tolerated, alternative drug therapy should be based on the patient’s cardiovascular risk profile.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient does not have CVD/is not at high CVD risk, consider using one of the following drugs instead of metformin:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
A dipeptidyl peptidase-4 (DPP-4) inhibitor , or
Pioglitazone, or
A sulfonylurea, or
An SGLT2 inhibitor (an SGLT2 inhibitor is recommended in some people: those in whom metformin is contraindicated or not tolerated, when diet and exercise alone do not provide adequate glycaemic control, only if a DPP-4 inhibitor would otherwise be prescribed and a sulfonylurea or pioglitazone is not appropriate).[152]National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes. May 2016 [internet publication].
https://www.nice.org.uk/guidance/ta390
[153]National Institute for Health and Care Excellence. Ertugliflozin as monotherapy or with metformin for treating type 2 diabetes. March 2019 [internet publication].
https://www.nice.org.uk/guidance/ta572
If the patient is at high risk of developing cardiovascular disease (aged ≥40 years with QRISK3 ≥10%, or aged <40 years with ≥1 cardiovascular risk factors), consider an SGLT2 inhibitor with proven cardiovascular benefit (e.g., canagliflozin, dapagliflozin, empagliflozin) in place of metformin.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient has chronic heart failure or established atherosclerotic CVD, give an SGLT2 inhibitor with proven cardiovascular benefit instead of metformin.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Before starting treatment with an SGLT2 inhibitor, always:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Check whether the patient is at an increased risk of diabetic ketoacidosis (DKA), for example:
They have had a previous episode of DKA
They are unwell with intercurrent illness
The patient is following a very low carbohydrate or ketogenic diet.
Advise the patient of the risk of DKA and address modifiable risk factors for DKA, for example, those who are following a very low carbohydrate or ketogenic diet, may need to delay treatment until they have changed their diet.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Drugs with cardiovascular and renal benefit
Unless metformin is contraindicated/not tolerated, as soon as metformin tolerability is confirmed (after gradually increasing the dose of immediate-release metformin over several weeks):[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient is at high risk of developing CVD, consider an SGLT2 inhibitor with proven cardiovascular benefit in addition to metformin.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient has chronic heart failure or established atherosclerotic CVD, add an SGLT2 inhibitor with proven cardiovascular benefit to their treatment regimen, that is, alongside metformin.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
These recommendations apply at all stages of treatment. Therefore, if a patient who did not originally have CVD develops CVD, add an SGLT2 inhibitor with proven cardiovascular benefit to their existing treatment regimen or give an SGLT2 inhibitor in place of an existing drug.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Likewise, if a patient is newly found to be at high risk of CVD, consider adding an SGLT2 inhibitor to their current treatments, or to switch one of their current drugs to an SGLT2 inhibitor.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Although NICE does not recommend GLP-1 receptor agonists as first-line treatment for type 2 diabetes, a 2022 update to a joint consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) recommends that monotherapy with a GLP-1 receptor agonist (e.g., dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide) or an SGLT2 inhibitor can be considered as an alternative to metformin, based on evidence regarding the cardiovascular and renal benefits of these drug classes, independent of their glucose-lowering effects.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[146]Li S, Vandvik PO, Lytvyn L, et al. SGLT-2 inhibitors or GLP-1 receptor agonists for adults with type 2 diabetes: a clinical practice guideline. BMJ. 2021 May 11;373:n1091.
https://www.bmj.com/content/373/bmj.n1091.long
http://www.ncbi.nlm.nih.gov/pubmed/33975892?tool=bestpractice.com
Specifically, ADA/EASD recommend:[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
A GLP-1 receptor agonist or an SGLT2 inhibitor with proven benefit should be used in people with established CVD, and should be considered in people without established CVD but with multiple cardiovascular risk factors. In people with heart failure, an SGLT2 inhibitor should be used.
In people with CKD and an eGFR ≥20 mL/min/1.73 m² and a urine ACR >3.0 mg/mmol (>30 mg/g), an SGLT2 inhibitor with proven benefit should be initiated. Indications and eGFR thresholds may vary by region. If such treatment is not tolerated or is contraindicated, a GLP-1 receptor agonist with proven cardiovascular outcome benefit could be considered and should be continued until kidney replacement therapy is indicated.
In people with heart failure, CKD, established CVD, or multiple risk factors for CVD, the decision to use GLP-1 receptor agonists or SGLT2 inhibitors with proven benefit should be independent of background use of metformin and of baseline HbA1c.
The ESC makes very similar recommendations to the ADA/EASD. The ESC recommends use of an SGLT2 inhibitor (with proven benefit) in patients with type 2 diabetes and CKD when eGFR is ≥20 mL/min/1.73 m² to reduce the risk of CVD and kidney failure.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Further, they recommend (independent of baseline/target HbA1c and any concomitant glucose-lowering medicine):[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
GLP-1 receptor agonists and SGLT2 inhibitors, with proven cardiovascular benefits, for patients with type 2 diabetes and ASCVD to reduce cardiovascular events. They further advise these same agents may be considered, as well as considering metformin, for those without ASCVD or severe TOD but with a 10-year CVD risk ≥10%, to reduce cardiovascular risk. They advise that treatment with metformin should not be a pre-requisite to use of these other agents for cardiovascular benefit.
SGLT2 inhibitors (with proven benefit) in patients with type 2 diabetes and heart failure (irrespective of ejection fraction), to reduce the risk of heart failure hospitalisation or cardiovascular death.[137]McDonagh TA, Metra M, Adamo M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023 Oct 1;44(37):3627-39.
https://academic.oup.com/eurheartj/article/44/37/3627/7246292?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622666?tool=bestpractice.com
SGLT2 inhibitors (with proven benefit) in patients with type 2 diabetes with multiple ASCVD risk factors or established ASCVD, to reduce the risk of heart failure hospitalisation.
Further, the ESC recommends that if additional glycaemic management is needed for patients with ASCVD following the use of antidiabetic medicines with proven cardiovascular benefits (e.g., SGLT2 inhibitors, GLP-1 receptor agonists), agents with proven cardiovascular safety should be prioritised over agents without evidence of cardiovascular benefit or safety.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The ESC also recommends that if additional glycaemic management is needed for patients with (or at risk of) heart failure following treatment with an SGLT2 inhibitor (with proven benefit), antidiabetic medicines with neutral effects on heart failure in cardiovascular outcome trials (CVOTs) should be considered.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
KDIGO notes that if additional glycaemic management is required in patients with type 2 diabetes and CKD following first-line treatment with metformin and SGLT2 inhibitors, GLP-1 receptor agonists are generally preferred.[135]Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int. 2022 Nov;102(5s):S1-127.
https://www.kidney-international.org/article/S0085-2538(22)00507-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36272764?tool=bestpractice.com
Similarly, the ESC recommends the use of a GLP-1 receptor agonist for additional glucose control (if required) in those with CKD and eGFR >15 mL/min/1.73 m² (given that these agents carry a low risk of hypoglycaemia and have beneficial effects on weight, cardiovascular risk, and albuminuria).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
The UKKA 2023 update suggests initiating an SGLT2 inhibitor for patients with type 2 diabetes and CKD (excluding kidney transplant recipients) with an eGFR of >45-60 mL/min/1.73 m² (and urinary albumin-to-creatinine ratio <25 mg/mmol) to modify cardiovascular risk and slow rate of kidney function decline (based on meta-analysis of large randomised clinical trials, showing that cardiovascular and renal benefits are present across a range of eGFR).[136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
They note that effects on glycaemic control may be limited, as the glucose lowering effects of SGLT2 inhibitors are reduced with declining eGFR. [136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
Antihyperglycaemic pharmacotherapy: escalation of therapy
NICE has produced a flowchart summarising its recommendations for choosing medicines for further treatment in adults with type 2 diabetes.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
NICE: Type 2 diabetes in adults: choosing medicines
Opens in new window
[Figure caption and citation for the preceding image starts]: Type 2 diabetes in adults: choosing medicines - how to choose medicines for further treatmentNICE NG28 (2022) Type 2 diabetes in adults: choosing medicines; used with permission [Citation ends].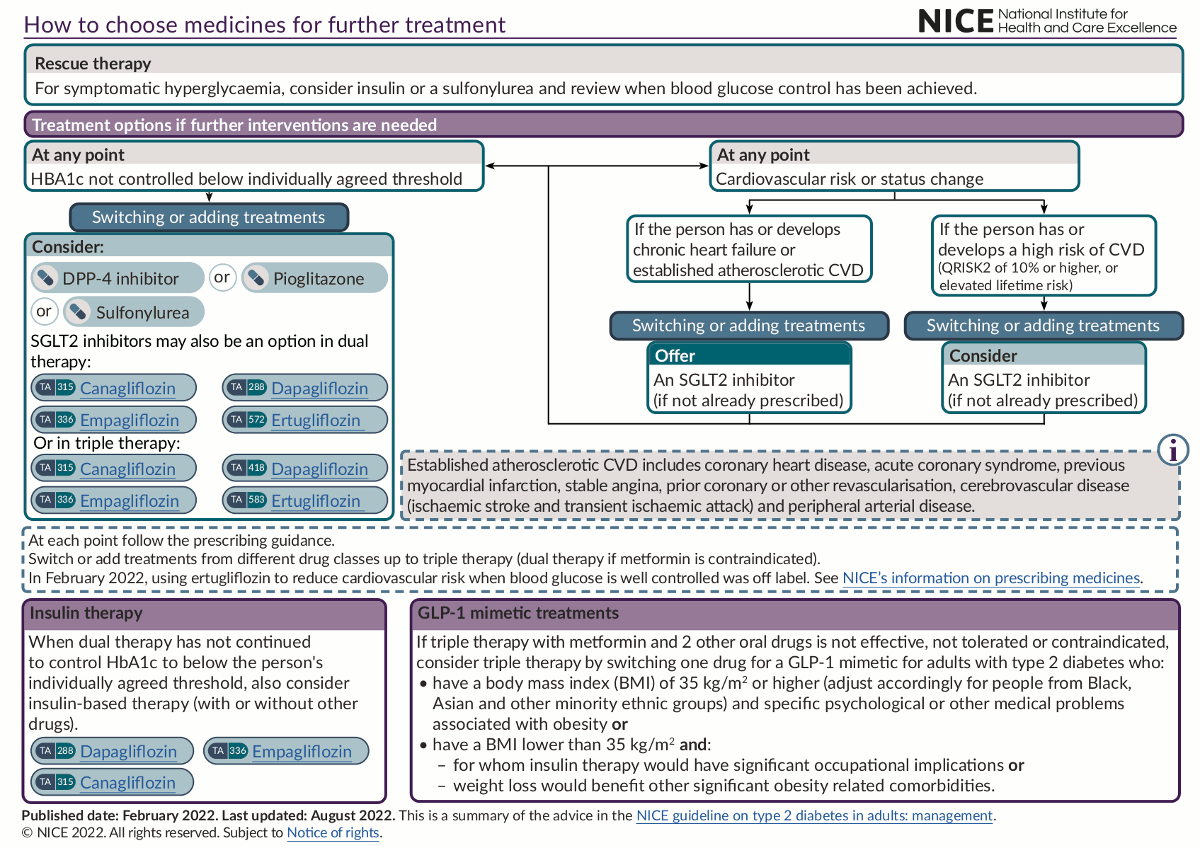
If the patient’s HbA1c is not controlled to below their individually agreed threshold, switching or adding treatments may be appropriate. Drugs should be introduced in a stepwise manner, checking for tolerability and effectiveness of each drug.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If initial therapy has not continued to control HbA1c to below the person's individually agreed threshold for further intervention, consider switching to, or adding:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
A DPP-4 inhibitor[153]National Institute for Health and Care Excellence. Ertugliflozin as monotherapy or with metformin for treating type 2 diabetes. March 2019 [internet publication].
https://www.nice.org.uk/guidance/ta572
[154]National Institute for Health and Care Excellence. Canagliflozin in combination therapy for treating type 2 diabetes. June 2014 [internet publication].
https://www.nice.org.uk/guidance/ta315
[155]National Institute for Health and Care Excellence. Empagliflozin in combination therapy for treating type 2 diabetes. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ta336
[156]National Institute for Health and Care Excellence. Dapagliflozin in combination therapy for treating type 2 diabetes. Nov 2016 [internet publication].
https://www.nice.org.uk/guidance/ta288
Pioglitazone
A sulfonylurea, or
An SGLT2-inhibitor.[154]National Institute for Health and Care Excellence. Canagliflozin in combination therapy for treating type 2 diabetes. June 2014 [internet publication].
https://www.nice.org.uk/guidance/ta315
[155]National Institute for Health and Care Excellence. Empagliflozin in combination therapy for treating type 2 diabetes. March 2015 [internet publication].
https://www.nice.org.uk/guidance/ta336
[156]National Institute for Health and Care Excellence. Dapagliflozin in combination therapy for treating type 2 diabetes. Nov 2016 [internet publication].
https://www.nice.org.uk/guidance/ta288
[157]National Institute for Health and Care Excellence. Ertugliflozin with metformin and a dipeptidyl peptidase-4 inhibitor for treating type 2 diabetes. Jun 2019 [internet publication].
https://www.nice.org.uk/guidance/TA583
If dual therapy with metformin and another oral drug has not continued to control HbA1c to below the patient’s individually agreed threshold for further intervention, consider either:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If metformin is contraindicated or not tolerated and dual therapy with 2 oral drugs has not continued to control HbA1c to below the patient's individually agreed threshold for intervention, consider insulin-based treatment (see Antihyperglycaemic pharmacotherapy: insulin-based treatments).[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If triple therapy with metformin and 2 other oral agents is not effective, not tolerated or contraindicated, consider triple therapy by switching one drug for a GLP-1 receptor agonist or tirzepatide (a dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist) in:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
Patients who have a BMI ≥35 kg/m² (adjust accordingly for people from black, Asian, and other minority ethnic groups) and specific psychological or other medical problems associated with obesity, or
Those with a BMI <35 kg/m² and for whom insulin therapy would have significant occupational implications, or weight loss would benefit other significant obesity-related comorbidities.
Of note, the ADA recommends that a GLP-1 receptor agonist or dual GIP/GLP-1 agonist is preferred over insulin when increased glycaemic management is required for adults with type 2 diabetes.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Warnings
Give careful consideration to the drug combinations, ensuring that they are safe and appropriate for your patient. If possible, avoid combining insulin with pioglitazone. The UK Medicines and Healthcare products Regulatory Agency (MHRA) warns that patients should be observed for signs and symptoms of heart failure, weight gain, and oedema if pioglitazone is used in combination with insulin. This is owing to the increased incidence of cardiac failure when pioglitazone is used in combination with insulin, especially in patients with predisposing factors.[160]Medicines and Healthcare products Regulatory Agency. Insulin combined with pioglitazone: risk of cardiac failure. December 2014 [internet publication].
https://www.gov.uk/drug-safety-update/insulin-combined-with-pioglitazone-risk-of-cardiac-failure
Only offer a GLP-1 receptor agonist in combination with insulin with specialist care advice and ongoing support from a consultant-led multi-disciplinary team.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The MHRA warns of cases of diabetic ketoacidosis in patients with type 2 diabetes on a combination of a GLP-1 receptor agonist and insulin, who had doses of concomitant insulin rapidly reduced or discontinued.[161]Medicines and Healthcare products Regulatory Agency. GLP-1 receptor agonists: reports of diabetic ketoacidosis when concomitant insulin was rapidly reduced or discontinued. June 2019 [internet publication].
https://www.gov.uk/drug-safety-update/glp-1-receptor-agonists-reports-of-diabetic-ketoacidosis-when-concomitant-insulin-was-rapidly-reduced-or-discontinued
The MHRA warns that the same risk cannot be excluded for the dual GIP/GLP-1 receptor agonist, tirzepatide, and appropriate caution should be taken if this is being used in combination with insulin.
Bear in mind that recommendations for drugs with cardiovascular and renal benefit apply at all stages of treatment. Therefore, if a patient who did not originally have CVD develops CVD, add an SGLT2 inhibitor with proven cardiovascular benefit to their existing treatment regimen or give an SGLT2 inhibitor in place of an existing drug.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Likewise, if a patient is newly found to be at high risk of CVD, consider adding an SGLT2 inhibitor to their current treatments, or to switch one of their current drugs to an SGLT2 inhibitor.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Clinical properties of specific antihyperglycaemic agents
Select agents on a case-by-case basis, following careful discussion with the patient of the pros and cons of each option. Agents that offer cardiovascular and renal protection, and reduce all-cause or cardiovascular mortality may be preferred.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Metformin
Metformin is an insulin ‘sensitiser’, which is given orally.[162]Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323.
https://www.doi.org/10.1093/eurheartj/ehz486
http://www.ncbi.nlm.nih.gov/pubmed/31497854?tool=bestpractice.com
It is highly effective at lowering blood glucose levels, it incurs minimal risk of hypoglycaemia when used as monotherapy, is weight neutral (with the potential for modest weight loss), has a good safety profile, and there is long-term experience of its use.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[163]Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021 Sep;23(9):2116-24.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14451
http://www.ncbi.nlm.nih.gov/pubmed/34047443?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
These properties, along with its favourable cost, mean that it is generally the recommended first choice agent in patients with type 2 diabetes (however, it should be noted that some guidelines such as those from the ESC in 2023 advocate use of other agents first line when the patient has specific additional risk factors or comorbidities such as ASCVD or CKD).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Metformin is likely to have beneficial effects on major adverse cardiovascular events (e.g., myocardial infarction, stroke, cardiovascular death) and all-cause mortality, and its long-term use is likely to improve cardiovascular prognosis (though the ESC advises that the cardiovascular benefits of metformin are inconclusive).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[142]UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998 Sep 12;352(9131):854-65.
http://www.ncbi.nlm.nih.gov/pubmed/9742977?tool=bestpractice.com
[143]Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577-89.
https://www.nejm.org/doi/full/10.1056/NEJMoa0806470
http://www.ncbi.nlm.nih.gov/pubmed/18784090?tool=bestpractice.com
[147]Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017 Sep;60(9):1620-9.
https://link.springer.com/article/10.1007%2Fs00125-017-4337-9
http://www.ncbi.nlm.nih.gov/pubmed/28770324?tool=bestpractice.com
[165]Monami M, Candido R, Pintaudi B, et al. Effect of metformin on all-cause mortality and major adverse cardiovascular events: an updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021 Mar 10;31(3):699-704.
https://www.nmcd-journal.com/article/S0939-4753(20)30508-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/33549430?tool=bestpractice.com
Metformin appears to have a neutral effect on heart failure and diabetic kidney disease.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Metformin may be safely used (with a possible dose reduction) in patients with reduced estimated glomerular filtration rates (eGFRs) with careful monitoring, but it is contraindicated if eGFR is <30 mL/min/1.73 m².[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[135]Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for diabetes management in chronic kidney disease. Kidney Int. 2022 Nov;102(5s):S1-127.
https://www.kidney-international.org/article/S0085-2538(22)00507-5/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36272764?tool=bestpractice.com
[166]de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and kidney disease: improving global outcomes (KDIGO). Diabetes Care. 2022 Dec 1;45(12):3075-90.
https://diabetesjournals.org/care/article/45/12/3075/147614/Diabetes-Management-in-Chronic-Kidney-Disease-A
http://www.ncbi.nlm.nih.gov/pubmed/36189689?tool=bestpractice.com
Gastrointestinal adverse effects are common with metformin.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Decreased/deficient vitamin B12 levels are also a recognised common side effect of treatment, especially in those receiving a higher dose or longer treatment duration and in those with existing risk factors.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[167]Medicines and Healthcare products Regulatory Agency. Metformin and reduced vitamin B12 levels: new advice for monitoring patients at risk. June 2022 [internet publication].
https://www.gov.uk/drug-safety-update/metformin-and-reduced-vitamin-b12-levels-new-advice-for-monitoring-patients-at-risk
SGLT2 inhibitors
SGLT2 inhibitors are renal glucose reuptake inhibitors.[162]Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323.
https://www.doi.org/10.1093/eurheartj/ehz486
http://www.ncbi.nlm.nih.gov/pubmed/31497854?tool=bestpractice.com
They are oral drugs, and examples include canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin.
They have an intermediate-to-high glucose lowering effect, with lower glycemic efficacy at lower eGFRs.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
They do not increase the risk of hypoglycaemia, and are associated with moderate weight loss (2-3 kg) in clinical practice.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[163]Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021 Sep;23(9):2116-24.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14451
http://www.ncbi.nlm.nih.gov/pubmed/34047443?tool=bestpractice.com
They cause renal glycosuria and have a diuretic effect, which reduces blood pressure.[168]Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015 Jan;75(1):33-59.
http://www.ncbi.nlm.nih.gov/pubmed/25488697?tool=bestpractice.com
There is also considerable evidence to support the benefits of specific SGLT2 inhibitors in reducing major adverse cardiac events, myocardial infarction, hospitalisation for heart failure, cardiovascular death, all-cause mortality, and improving renal outcomes, in patients with type 2 diabetes with established/high-risk of CVD.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[169]Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021 Jan 13;372:m4573.
https://www.bmj.com/content/372/bmj.m4573.long
http://www.ncbi.nlm.nih.gov/pubmed/33441402?tool=bestpractice.com
The benefits are largely independent of their glucose lowering effect.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Specifically, canagliflozin and empagliflozin have been shown to have beneficial effects on major adverse cardiac events and cardiovascular mortality; canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin have shown beneficial effects on heart failure; and canagliflozin, dapagliflozin, and empagliflozin have shown beneficial renal effects including on the progression of diabetic kidney disease and preventing major kidney outcomes (dialysis, transplantation, or death due to kidney disease).[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[170]Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019 Apr 23;139(17):2022-31.
http://www.ncbi.nlm.nih.gov/pubmed/30786725?tool=bestpractice.com
[171]Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017 Aug 17;377(7):644-57.
http://www.nejm.org/doi/full/10.1056/NEJMoa1611925#t=article
http://www.ncbi.nlm.nih.gov/pubmed/28605608?tool=bestpractice.com
[172]Cherney DZ, Zinman B, Inzucchi SE, et al. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017 Aug;5(8):610-21.
http://www.ncbi.nlm.nih.gov/pubmed/28666775?tool=bestpractice.com
[173]Mahaffey KW, Jardine MJ, Bompoint S, et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019 Aug 27;140(9):739-50.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.119.042007
http://www.ncbi.nlm.nih.gov/pubmed/31291786?tool=bestpractice.com
[174]Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019 Jan 5;393(10166):31-39.
http://www.ncbi.nlm.nih.gov/pubmed/30424892?tool=bestpractice.com
[175]Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019 Jun 13;380(24):2295-306.
https://www.nejm.org/doi/10.1056/NEJMoa1811744
http://www.ncbi.nlm.nih.gov/pubmed/30990260?tool=bestpractice.com
[176]Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28.
https://www.nejm.org/doi/10.1056/NEJMoa1504720?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/26378978?tool=bestpractice.com
[177]Mahaffey KW, Neal B, Perkovic V, et al; CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018 Jan 23;137(4):323-34.
https://www.ahajournals.org/doi/full/10.1161/circulationaha.117.032038
http://www.ncbi.nlm.nih.gov/pubmed/29133604?tool=bestpractice.com
[178]Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation. 2018 Jul 31;138(5):458-68.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.118.034222
http://www.ncbi.nlm.nih.gov/pubmed/29526832?tool=bestpractice.com
[179]Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019 Jan 24;380(4):347-57.
https://www.nejm.org/doi/full/10.1056/NEJMoa1812389
http://www.ncbi.nlm.nih.gov/pubmed/30415602?tool=bestpractice.com
[180]Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019 Nov;7(11):845-54.
http://www.ncbi.nlm.nih.gov/pubmed/31495651?tool=bestpractice.com
Data analysis suggests that SGLT2 inhibitors have beneficial effects on heart failure-related outcomes in people with heart failure, irrespective of ejection fraction or diabetes status.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[181]Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019 May 28;139(22):2528-36.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.040130
http://www.ncbi.nlm.nih.gov/pubmed/30882238?tool=bestpractice.com
[182]Figtree GA, Rådholm K, Barrett TD, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. 2019 May 28;139(22):2591-3.
https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.040057
http://www.ncbi.nlm.nih.gov/pubmed/30882240?tool=bestpractice.com
[183]McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019 Nov 21;381(21):1995-2008.
https://www.nejm.org/doi/10.1056/NEJMoa1911303
http://www.ncbi.nlm.nih.gov/pubmed/31535829?tool=bestpractice.com
[184]National Institute for Health and Care Excellence. Dapagliflozin for treating chronic heart failure with reduced ejection fraction. February 2021 [internet publication].
https://www.nice.org.uk/guidance/TA679
[185]Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022 Sep 3;400(10354):757-67.
https://pubmed.ncbi.nlm.nih.gov/36041474
http://www.ncbi.nlm.nih.gov/pubmed/36041474?tool=bestpractice.com
[186]Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020 Oct 8;383(15):1413-24.
https://www.nejm.org/doi/10.1056/NEJMoa2022190?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32865377?tool=bestpractice.com
[187]Chen C, Peng H, Li M, et al. Patients with type 2 diabetes mellitus and heart failure benefit more from sodium-glucose cotransporter 2 inhibitor: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2021 Oct 25:12:664533.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8572881
http://www.ncbi.nlm.nih.gov/pubmed/34759887?tool=bestpractice.com
In addition, a meta-analysis of randomised-controlled trial data showed that SGLT2 inhibitors reduce the risk of serious hyperkalemia in people with type 2 diabetes at high cardiovascular risk or with chronic kidney disease, without increasing the risk of hypokalaemia.[188]Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2 inhibitors and risk of hyperkalemia in people with type 2 diabetes: a meta-analysis of individual participant data from randomized, controlled trials. Circulation. 2022 May 10;145(19):1460-70.
https://www.ahajournals.org/doi/full/10.1161/CIRCULATIONAHA.121.057736?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
http://www.ncbi.nlm.nih.gov/pubmed/35394821?tool=bestpractice.com
To date, there is greater uncertainty around the cardiovascular benefits associated with ertugliflozin than for the other SGLT2 inhibitors.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
[189]Cinti F, Moffa S, Impronta F, et al. Spotlight on ertugliflozin and its potential in the treatment of type 2 diabetes: evidence to date. Drug Des Devel Ther. 2017 Oct 3;11:2905-19.
https://www.dovepress.com/spotlight-on-ertugliflozin-and-its-potential-in-the-treatment-of-type--peer-reviewed-fulltext-article-DDDT
http://www.ncbi.nlm.nih.gov/pubmed/29042751?tool=bestpractice.com
In their guideline on type 2 diabetes, NICE advised that ertugliflozin did not show consistent reduction of heart failure versus placebo in network meta-analysis, nor was it statistically significantly better than placebo for 3-point major adverse cardiovascular events (MACE) outcome (comprising cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke).[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Further, one randomised double-blind phase 3 trial assessed the effects of ertugliflozin versus placebo on cardiovascular and renal outcomes in people with type 2 diabetes and established atherosclerotic cardiovascular disease; results showed non-inferiority to placebo in the primary outcome of major adverse cardiovascular events, but no superiority in key secondary cardiovascular and renal outcomes.[190]ClinicalTrials.gov. Cardiovascular outcomes following ertugliflozin treatment in type 2 diabetes mellitus participants with vascular disease: the VERTIS CV study (MK-8835-004). April 2019 [internet publication].
https://clinicaltrials.gov/ct2/show/NCT01986881
[191]Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020 Oct 8;383(15):1425-35.
https://www.nejm.org/doi/10.1056/NEJMoa2004967?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32966714?tool=bestpractice.com
SGLT-2 inhibitors are associated with an increased risk of mycotic genital infections (usually mild and treatable), and an increased risk of diabetic ketoacidosis (although the incidence is low).[73]Medicines and Healthcare products Regulatory Agency. SGLT2 inhibitors: updated advice on the risk of diabetic ketoacidosis. April 2016 [internet publication].
https://www.gov.uk/drug-safety-update/sglt2-inhibitors-updated-advice-on-the-risk-of-diabetic-ketoacidosis
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[192]Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018 Nov 14;363:k4365.
https://www.bmj.com/content/363/bmj.k4365.long
http://www.ncbi.nlm.nih.gov/pubmed/30429124?tool=bestpractice.com
[193]Erondu N, Desai M, Ways K, et al. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015 Sep;38(9):1680-6.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4542268
http://www.ncbi.nlm.nih.gov/pubmed/26203064?tool=bestpractice.com
[194]Peters AL, Buschur EO, Buse JB, et al. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015 Sep;38(9):1687-93.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4542270
http://www.ncbi.nlm.nih.gov/pubmed/26078479?tool=bestpractice.com
[195]Fleming N, Hamblin PS, Story D, et al. Evolving evidence of diabetic ketoacidosis in patients taking sodium-glucose cotransporter 2 inhibitors. J Clin Endocrinol Metab. 2020 Aug 1;105(8):dgaa200.
https://academic.oup.com/jcem/article/105/8/2475/5821255?login=false
http://www.ncbi.nlm.nih.gov/pubmed/32302001?tool=bestpractice.com
The US Food and Drug Administration (FDA) has issued a warning of the risk of acute kidney injury with canagliflozin and dapagliflozin.[196]US Food and Drug Administration (FDA). FDA drug safety communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR). June 2016 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-strengthens-kidney-warnings-diabetes-medicines-canagliflozin
The MHRA and European Medicines Agency (EMA) warn of an increased risk of lower-limb amputation (mainly toes) in patients with type 2 diabetes taking canagliflozin, and the MHRA emphasises the importance of preventive foot care for all patients with diabetes.[197]European Medicines Agency. SGLT2 inhibitors: information on potential risk of toe amputation to be included in prescribing information. February 2017 [internet publication].
https://www.ema.europa.eu/en/documents/referral/sglt2-inhibitors-previously-canagliflozin-article-20-procedure-sglt2-inhibitors-information_en-0.pdf
[198]Medicines and Healthcare products Regulatory Agency. SGLT2 inhibitors: updated advice on increased risk of lower-limb amputation (mainly toes). March 2017 [internet publication].
https://www.gov.uk/drug-safety-update/sglt2-inhibitors-updated-advice-on-increased-risk-of-lower-limb-amputation-mainly-toes
Therefore, patients with foot ulcers or at high risk for amputation should be given comprehensive foot-care education while treating with SGLT2 inhibitors. The EASD/ADA guidelines on the management of hyperglycaemia in type 2 diabetes notes that while early studies highlighted potential SGLT2 inhibitor-related safety concerns, including acute kidney injury and amputation, longer-term studies that have prospectively assessed and monitored these events have not seen a significant imbalance in risks.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[199]Dorsey-Treviño EG, González-González JG, Alvarez-Villalobos N, et al. Sodium-glucose cotransporter 2 (SGLT-2) inhibitors and microvascular outcomes in patients with type 2 diabetes: systematic review and meta-analysis. J Endocrinol Invest. 2020 Mar;43(3):289-304.
http://www.ncbi.nlm.nih.gov/pubmed/31489568?tool=bestpractice.com
The MHRA and FDA also warn of cases of necrotising fasciitis of the perineum (also known as Fournier's gangrene) observed in post-marketing surveillance of SGLT2 inhibitors.[200]US Food and Drug Administration. FDA drug safety communication: FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. August 2018 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes
[201]Medicines and Healthcare products Regulatory Agency. SGLT2 inhibitors: reports of Fournier’s gangrene (necrotising fasciitis of the genitalia or perineum). February 2019 [internet publication].
https://www.gov.uk/drug-safety-update/sglt2-inhibitors-reports-of-fournier-s-gangrene-necrotising-fasciitis-of-the-genitalia-or-perineum
GLP-1 receptor agonists
GLP-1 receptor agonists are incretin-based drugs.[162]Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323.
https://www.doi.org/10.1093/eurheartj/ehz486
http://www.ncbi.nlm.nih.gov/pubmed/31497854?tool=bestpractice.com
Incretins are a group of metabolic hormones that decrease blood glucose levels by stimulating the release of insulin and inhibiting the release of glucagon; the two most important incretins are the intestinal peptides GLP-1 and GIP. GLP-1 receptor agonists promote glucose-dependent insulin secretion and glucagon suppression, decelerate gastric emptying, limit post-meal glycemic increments, and reduce appetite and energy intake.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Examples include dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide. They are usually administered subcutaneously, although there is an oral formulation of semaglutide available.
They have a high/very high glucose-lowering effect and do not cause hypoglycaemia.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
Evidence supports the benefits of specific GLP-1 receptor agonists in reducing major adverse cardiac events and progression of diabetic kidney disease in high-risk patients with type 2 diabetes; these benefits largely occur independently of their glucose-lowering effects.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[169]Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021 Jan 13;372:m4573.
https://www.bmj.com/content/372/bmj.m4573.long
http://www.ncbi.nlm.nih.gov/pubmed/33441402?tool=bestpractice.com
[202]Giugliano D, Scappaticcio L, Longo M, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021 Sep 15;20(1):189.
https://cardiab.biomedcentral.com/articles/10.1186/s12933-021-01366-8
http://www.ncbi.nlm.nih.gov/pubmed/34526024?tool=bestpractice.com
For example, dulaglutide and semaglutide have both been shown to reduce major cardiovascular events, but not all-cause or cardiovascular mortality.[203]Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019 Jul 13;394(10193):121-30.
http://www.ncbi.nlm.nih.gov/pubmed/31189511?tool=bestpractice.com
[204]Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016 Nov 10;375(19):1834-44.
http://www.nejm.org/doi/full/10.1056/NEJMoa1607141#t=article
http://www.ncbi.nlm.nih.gov/pubmed/27633186?tool=bestpractice.com
[205]Kaul S. Mitigating cardiovascular risk in type 2 diabetes with antidiabetes drugs: a review of principal cardiovascular outcome results of EMPA-REG OUTCOME, LEADER, and SUSTAIN-6 trials. Diabetes Care. 2017 Jul;40(7):821-31.
https://care.diabetesjournals.org/content/40/7/821.long
http://www.ncbi.nlm.nih.gov/pubmed/28637887?tool=bestpractice.com
Liraglutide significantly reduced cardiovascular mortality and all-cause mortality in those with diabetes and cardiovascular disease or high CVD risk in one randomised trial.[206]Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee on behalf of the LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016 Jul 28;375(4):311-22.
http://www.nejm.org/doi/full/10.1056/NEJMoa1603827#t=article
http://www.ncbi.nlm.nih.gov/pubmed/27295427?tool=bestpractice.com
However, trials have shown that exenatide and lixisenatide do not reduce major cardiovascular events.[207]Hu Y. Advances in reducing cardiovascular risk in the management of patients with type 2 diabetes mellitus. Chronic Dis Transl Med. 2019 Mar 15;5(1):25-36.
https://www.sciencedirect.com/science/article/pii/S2095882X18300653
http://www.ncbi.nlm.nih.gov/pubmed/30993261?tool=bestpractice.com
Dulaglutide, liraglutide, and semaglutide (subcutaneous) have been shown to produce beneficial renal endpoints, driven by albuminuria outcomes.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[208]Yuan D, Sharma H, Krishnan A, et al. Effect of glucagon-like peptide 1 receptor agonists on albuminuria in adult patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab. 2022 Sep;24(9):1869-81.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14776
http://www.ncbi.nlm.nih.gov/pubmed/35589615?tool=bestpractice.com
A meta-analysis of randomised placebo-controlled trials studying GLP-1 receptor agonists in patients with type 2 diabetes showed that treatment did not reduce heart-failure hospitalisations and mortality in patients with heart failure, but that it may prevent new-onset heart failure and mortality in patients without heart failure.[209]Ferreira JP, Saraiva F, Sharma A, et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes Metab. 2023 Jun;25(6):1495-502.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14997
http://www.ncbi.nlm.nih.gov/pubmed/36722252?tool=bestpractice.com
The reduction in atherosclerotic events observed with GLP-1 receptor agonists was not influenced by heart-failure status.[209]Ferreira JP, Saraiva F, Sharma A, et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes Metab. 2023 Jun;25(6):1495-502.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14997
http://www.ncbi.nlm.nih.gov/pubmed/36722252?tool=bestpractice.com
GLP-1 receptor agonists produce intermediate to very high weight loss.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[163]Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021 Sep;23(9):2116-24.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14451
http://www.ncbi.nlm.nih.gov/pubmed/34047443?tool=bestpractice.com
They are suitable for obese patients without gastroparesis who desire weight loss, are willing to take injections, and can tolerate the common adverse effect of initial nausea.[210]Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab. 2017 Apr;19(4):524-36.
http://www.ncbi.nlm.nih.gov/pubmed/27981757?tool=bestpractice.com
In one review, GLP-1 receptor agonist use led to loss of 1.4 kg versus placebo, and loss of 4.8 kg versus insulin.[211]Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007 Jul 11;298(2):194-206.
http://www.ncbi.nlm.nih.gov/pubmed/17622601?tool=bestpractice.com
A systematic review and meta-analysis of liraglutide indicates it produced significant weight reduction with a reasonable safety profile for patients who have overweight or obesity regardless of diabetic status compared to placebo.[212]Konwar M, Bose D, Jaiswal SK, et al. Efficacy and safety of liraglutide 3.0 mg in patients with overweight and obese with or without diabetes: a systematic review and meta-analysis. Int J Clin Pract. 2022 Jul 19:2022:1201977.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9325632
http://www.ncbi.nlm.nih.gov/pubmed/35936066?tool=bestpractice.com
The ADA recommends that for adults with diabetes and overweight or obesity, a GLP-1 receptor agonist with greater weight loss efficacy (i.e., semaglutide) is a preferred option where pharmacotherapy is indicated as an adjunct to lifestyle changes for weight management.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
They advise that this approach has added weight-independent benefits (e.g., glycaemic and cardiometabolic).[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
GLP-1 receptor agonists are effective at improving steatosis and some randomised controlled trials have demonstrated potential benefits with liraglutide and semaglutide in patients with metabolic dysfunction-associated steatohepatitis (MASH; formerly known as non-alcoholic steatohepatitis [NASH]), including notably slowing of liver fibrosis progression.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
The ADA recommends considering the use of a GLP-1 receptor agonist (with demonstrated benefit in MASH, formerly known as NASH) for adults with type 2 diabetes (particularly those with overweight or obesity) and metabolic dysfunction-associated steatotic liver disease (MASLD, formerly known as non-alcoholic fatty liver disease [NAFLD]), as an adjunct to lifestyle interventions for weight loss.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
The ADA also recommends that GLP-1 receptor agonists are a preferred option for hyperglycaemia treatment in people with type 2 diabetes with biopsy-confirmed MASH (formerly NASH) or at high risk with clinically significant liver fibrosis on non-invasive testing.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
The most common adverse effects of GLP-1 inhibitors are gastrointestinal, such as nausea, vomiting, and diarrhoea, but they tend to reduce over time. In addition, the MHRA warns of cases of diabetic ketoacidosis in patients with type 2 diabetes on a combination of a GLP-1 receptor agonist and insulin who had doses of concomitant insulin rapidly reduced or discontinued.[161]Medicines and Healthcare products Regulatory Agency. GLP-1 receptor agonists: reports of diabetic ketoacidosis when concomitant insulin was rapidly reduced or discontinued. June 2019 [internet publication].
https://www.gov.uk/drug-safety-update/glp-1-receptor-agonists-reports-of-diabetic-ketoacidosis-when-concomitant-insulin-was-rapidly-reduced-or-discontinued
Pancreatitis has been reported in clinical trials of GLP-1 receptor agonists, but the causality has not been established.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
There is conflicting evidence regarding whether GLP-1 receptor agonists increase the risk of thyroid disorders.
A 2022 meta-analysis of randomised-controlled trials concluded that GLP-1 receptor agonists did not increase or decrease the risk of thyroid cancer, hyperthyroidism, hypothyroidism, thyroiditis, thyroid mass, and goiter, although it noted that due to the low incidence of these diseases, these findings needed to be examined further.[213]Hu W, Song R, Cheng R, et al. Use of GLP-1 Receptor agonists and occurrence of thyroid disorders: a meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). 2022 Jul 11:13:927859.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9309474
http://www.ncbi.nlm.nih.gov/pubmed/35898463?tool=bestpractice.com
However, a nested case-control analysis of 2562 subjects with thyroid cancer reported that GLP-1 receptor agonist use was associated with an increased risk all thyroid cancer and medullary thyroid cancer, in particular after 1-3 years of treatment.[214]Bezin J, Gouverneur A, Pénichon M, et al. GLP-1 receptor agonists and the risk of thyroid cancer. Diabetes Care. 2023 Feb 1;46(2):384-90.
https://diabetesjournals.org/care/article-abstract/46/2/384/147888/GLP-1-Receptor-Agonists-and-the-Risk-of-Thyroid?redirectedFrom=fulltext
http://www.ncbi.nlm.nih.gov/pubmed/36356111?tool=bestpractice.com
A systematic review and network meta-analysis combining 84 trials investigating the effect of GLP-1 receptor agonists and DPP-4 inhibitors (incretin-based therapies) concluded that they were not associated with an increased risk of cancers of the digestive system in patients with type 2 diabetes.[215]Chai S, Yu S, Yang Z, et al. Effect of incretin-based therapies on cancers of digestive system among 101 595 patients with type 2 diabetes mellitus: a systematic review and network meta-analysis combining 84 trials with a median duration of 30 weeks. BMJ Open Diabetes Res Care. 2019 Sep 20;7(1):e000728.
https://drc.bmj.com/content/7/1/e000728.long
http://www.ncbi.nlm.nih.gov/pubmed/31641525?tool=bestpractice.com
The EMA is reviewing data on the risk of suicidal thoughts and thoughts of self-harm with GLP-1 receptor agonists, following reports of such occurrences in people using liraglutide and semaglutide.
Dual GIP/GLP-1 receptor agonist
Tirzepatide is the first and only dual GIP/GLP-1 receptor agonist available. It increases insulin sensitivity and secretion, suppresses glucagon secretion, and slows gastric emptying. In 2023, NICE recommended tirzepatide (as an adjunct to diet and exercise) for use in adults whose type 2 diabetes is inadequately controlled, only if triple antidiabetic therapy is ineffective, contraindicated, or not tolerated, and they fulfil one of the following criteria:[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
BMI ≥35 kg/m² (adjust accordingly for people from black, Asian, and other minority ethnic groups) and specific psychological or other medical problems associated with obesity; or
BMI <35 kg/m² and significant occupational implications of insulin therapy or potential benefits of weight loss on other significant obesity-related complications.
NICE positions tirzepatide as an alternative to using a GLP-1 receptor agonist (i.e., tirzepatide is an option to be considered at the same level of the UK type 2 diabetes treatment pathway as GLP-1 receptor agonists).[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
NICE notes that tirzepatide is used earlier in the treatment pathway internationally, but in their review it was only considered as an alternative option to starting a GLP-1 receptor agonist.[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
Tirzepatide is approved in Europe for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise (as monotherapy when metformin is considered inappropriate due to intolerance or contraindications; or in addition to other medicinal products for the treatment of diabetes). American guidance on type 2 diabetes in adults from the ADA includes recommendations for a dual GIP/GLP-1 receptor agonist (i.e., tirzepatide) as a preferred option where pharmacotherapy is indicated for weight management in those with overweight or obesity (the ADA emphasises that this approach has added weight-independent glycaemic and cardiometabolic benefits).[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Tirzepatide has been shown to have a greater effect on glucose levels and weight control than selective GLP-1 receptor agonists alone, without increased risk of hypoglycaemia.[216]Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021 Jul 10;398(10295):143-55.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01324-6/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34186022?tool=bestpractice.com
[217]Frías JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021 Aug 5;385(6):503-15.
https://www.nejm.org/doi/10.1056/NEJMoa2107519?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/34170647?tool=bestpractice.com
[218]Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018 Nov 17;392(10160):2180-93.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)32260-8/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30293770?tool=bestpractice.com
[219]Inagaki N, Takeuchi M, Oura T, et al. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J-mono): a double-blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022 Sep;10(9):623-33.
https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00188-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/35914543?tool=bestpractice.com
Trials have shown that in patients with type 2 diabetes, tirzepatide was superior to titrated insulin degludec (with greater reductions in HbA1c and bodyweight and a lower risk of hypoglycaemia), and in patients with type 2 diabetes and an elevated cardiovascular risk, tirzepatide was superior to insulin glargine (with greater and clinically meaningful HbA1c reduction with a lower incidence of hypoglycaemia).[220]Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021 Aug 14;398(10300):583-98.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)01443-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34370970?tool=bestpractice.com
[221]Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-24.
http://www.ncbi.nlm.nih.gov/pubmed/34672967?tool=bestpractice.com
NICE notes that higher doses of tirzepatide are associated with greater degree of weight loss, whereas the effects on HbA1c seem to be less dose-dependent, present even at low doses.[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
Analyses (including a pre-specified meta-analysis) of initial results on the short-term cardiovascular safety of tirzepatide from the SURPASS clinical trials conducted in patients with type 2 diabetes, suggest no excess cardiovascular risk or increase in the risk of major cardiovascular events with tirzepatide when compared with controls (including semaglutide and dulaglutide, GLP-1 receptor agonists with known cardiovascular benefits).[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[221]Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021 Nov 13;398(10313):1811-24.
http://www.ncbi.nlm.nih.gov/pubmed/34672967?tool=bestpractice.com
[222]Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat Med. 2022 Mar;28(3):591-8.
https://www.nature.com/articles/s41591-022-01707-4
http://www.ncbi.nlm.nih.gov/pubmed/35210595?tool=bestpractice.com
Additionally, post-hoc analysis of the clinical trial SURMOUNT-1 (conducted to assess the efficacy and safety of tirzepatide in patients with obesity or overweight), which notably excluded patients with type 2 diabetes, concluded that significant benefits in cardiovascular risk reduction were seen with tirzepatide versus placebo.[223]Hankosky ER, Wang H, Neff LM, et al. Tirzepatide reduces the predicted risk of atherosclerotic cardiovascular disease and improves cardiometabolic risk factors in adults with obesity or overweight: SURMOUNT-1 post hoc analysis. Diabetes Obes Metab. 2024 Jan;26(1):319-28.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.15318
http://www.ncbi.nlm.nih.gov/pubmed/37932236?tool=bestpractice.com
However, further research on the long-term cardiovascular effects of tirzepatide is required. The ongoing trials SURPASS-CVOT (in patients with type 2 diabetes) and SURMOUNT-MMO (in patients with obesity without diabetes) will look to provide more robust data on the cardiovascular safety of tirzepatide and its effects on cardiovascular outcomes and morbidity and mortality.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[224]Nicholls SJ, Bhatt DL, Buse JB, et al. Comparison of tirzepatide and dulaglutide on major adverse cardiovascular events in participants with type 2 diabetes and atherosclerotic cardiovascular disease: SURPASS-CVOT design and baseline characteristics. Am Heart J. 2024 Jan;267:1-11.
https://www.sciencedirect.com/science/article/pii/S0002870323002806?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/37758044?tool=bestpractice.com
[225]ClinicalTrials.gov. A study of tirzepatide (LY3298176) on the reduction on morbidity and mortality in adults with obesity (SURMOUNT-MMO). ClinicalTrials.gov Identifier: NCT05556512. Oct 2024 [internet publication].
https://clinicaltrials.gov/study/NCT05556512
Post-hoc analysis of SURPASS-4, which compared tirzepatide with insulin glargine in patients with type 2 diabetes and increased cardiovascular risk or established CVD, suggested tirzepatide may be associated with renal benefits.[226]Heerspink HJL, Sattar N, Pavo I, et al. Effects of tirzepatide versus insulin glargine on kidney outcomes in type 2 diabetes in the SURPASS-4 trial: post-hoc analysis of an open-label, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022 Nov;10(11):774-85.
http://www.ncbi.nlm.nih.gov/pubmed/36152639?tool=bestpractice.com
Tirzepatide was reasonably well tolerated in trials, with the most common adverse effects being similar to those seen with GLP-1 receptor agonists (nausea, vomiting, and dyspepsia). Slow uptitration of the dose of tirzepatide may minimise the risk of these adverse effects.[159]National Institute for Health and Care Excellence. Tirzepatide for treating type 2 diabetes. Oct 2023 [internet publication].
https://www.nice.org.uk/guidance/ta924/chapter/1-Recommendations
The same warnings and cautions noted above for GLP-1 receptor agonists apply to tirzepatide.
DPP-4 inhibitors
DPP-4 inhibitors are incretin-based oral drugs.[162]Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323.
https://www.doi.org/10.1093/eurheartj/ehz486
http://www.ncbi.nlm.nih.gov/pubmed/31497854?tool=bestpractice.com
The incretins GLP-1 and GIP are rapidly inactivated by the DPP-4 enzyme. DPP-4 inhibitors (also known as gliptins) include alogliptin, linagliptin, saxagliptin, sitagliptin, and vildagliptin. They have a modest glucose-lowering effect, are weight-neutral, and are generally well tolerated with a minimal risk of hypoglycaemia.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[163]Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021 Sep;23(9):2116-24.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14451
http://www.ncbi.nlm.nih.gov/pubmed/34047443?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
Overall, DPP-4 inhibitors are not associated with any increase or reduction of major adverse cardiac events, all-cause mortality, or heart failure; however, saxagliptin may be associated with an increased risk of hospitalisation for heart failure (and is therefore not recommended by the ESC for use in patients at risk of or with a history of heart failure).[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[227]Mannucci E, Nreu B, Montereggi C, et al. Cardiovascular events and all-cause mortality in patients with type 2 diabetes treated with dipeptidyl peptidase-4 inhibitors: an extensive meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021 Sep 22;31(10):2745-55.
https://www.nmcd-journal.com/article/S0939-4753(21)00279-9/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/34364771?tool=bestpractice.com
Vildagliptin, the only DPP-4 inhibitor mentioned above not studied in a dedicated CVOT, was found to be associated with increased left ventricular volumes (of unknown clinical significance) in a small trial, but no significant effect on left ventricular ejection fraction (LVEF) was demonstrated.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
[228]McMurray JJV, Ponikowski P, Bolli GB, et al. Effects of vildagliptin on ventricular function in patients with type 2 diabetes mellitus and heart failure: a randomized placebo-controlled trial. JACC Heart Fail. 2018 Jan;6(1):8-17.
https://www.sciencedirect.com/science/article/pii/S2213177917305358?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/29032139?tool=bestpractice.com
For patients with type 2 diabetes with or at risk of heart failure, guidelines from the ESC recommend that linagliptin and sitagliptin have a neutral effect on the risk of hospitalisation for heart failure and should be considered as options for glucose-lowering in these patients.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
DPP-4 inhibitors do not delay the progression of diabetic kidney disease; however, a reduction in the risk of albuminuria progression was noted with linagliptin in a placebo-controlled trial.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
DPP-4 inhibitors can be used in cases of renal impairment, although dose adjustment may be required.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Joint pain is a potential rare adverse event.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Pancreatitis has been reported in clinical trials, but causality has not been established; the drugs should be discontinued if pancreatitis is suspected. Recent analyses have suggested that DPP-4 inhibitors are associated with an increased risk of acute liver injury (compared with SGLT-2 inhibitors) in patients with type 2 diabetes.[229]Pradhan R, Yin H, Yu OHY, et al. Incretin-based drugs and the risk of acute liver injury among patients with type 2 diabetes. Diabetes Care. 2022 Oct 1;45(10):2289-98.
https://diabetesjournals.org/care/article/45/10/2289/147257/Incretin-Based-Drugs-and-the-Risk-of-Acute-Liver
http://www.ncbi.nlm.nih.gov/pubmed/35866685?tool=bestpractice.com
In addition, the FDA adverse event reporting system noted that there were consistent signals of gallbladder- or biliary-related events associated with DPP-4 inhibitors with real-world data, and that clinicians should be aware of these in clinical practice.[230]He L, Wang J, Li Z, et al. Dipeptidyl peptidase 4 inhibitors and gallbladder or biliary diseases: data from the U.S. Food and Drug Administration adverse event reporting system. Diabetes Care. 2023 Feb 1;46(2):e72-3.
https://diabetesjournals.org/care/article/46/2/e72/148158/Dipeptidyl-Peptidase-4-Inhibitors-and-Gallbladder
http://www.ncbi.nlm.nih.gov/pubmed/36534433?tool=bestpractice.com
Sulfonylureas
Sulfonylureas are oral drugs that stimulate the release of insulin from pancreatic beta cells. Examples include gliclazide and glimepiride.
Sulfonylureas have a high glucose-lowering effect, and are the subject of long clinical experience.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
They may reduce microvascular complications, but do not provide cardiovascular risk reduction or delay the progression of diabetic kidney disease, and may cause weight gain and hypoglycaemia.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[163]Tsapas A, Karagiannis T, Kakotrichi P, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021 Sep;23(9):2116-24.
https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/dom.14451
http://www.ncbi.nlm.nih.gov/pubmed/34047443?tool=bestpractice.com
Adverse cardiovascular outcomes have been reported in some studies, although systematic reviews have not found an increase in all-cause mortality compared with other active treatments.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[231]Khunti K, Chatterjee S, Gerstein HC, et al. Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol. 2018 Oct;6(10):821-32.
https://www.thelancet.com/journals/landia/article/PIIS2213-8587(18)30025-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/29501322?tool=bestpractice.com
Furthermore, a randomised-controlled trial in adults with type 2 diabetes showed comparable cardiovascular safety of the sulfonylurea, glimepiride, compared with the DPP-4 inhibitor, linagliptin, over 6.3 years, and a Scottish real-world comparative safety study concluded that second-line sulfonylureas are unlikely to increase cardiovascular risk or all-cause mortality.[232]Rosenstock J, Kahn SE, Johansen OE, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019 Sep 24;322(12):1155-66.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6763993
http://www.ncbi.nlm.nih.gov/pubmed/31536101?tool=bestpractice.com
[233]Wang H, Cordiner RLM, Huang Y, et al. Cardiovascular safety in type 2 diabetes with sulfonylureas as second-line drugs: a nationwide population-based comparative safety study. Diabetes Care. 2023 May 1;46(5):967-77.
https://diabetesjournals.org/care/article/46/5/967/148656/Cardiovascular-Safety-in-Type-2-Diabetes-With
http://www.ncbi.nlm.nih.gov/pubmed/36944118?tool=bestpractice.com
They should be used with caution in people at risk of hypoglycaemia.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Pioglitazone
Pioglitazone is an oral drug that is an insulin ‘sensitiser’.[162]Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323.
https://www.doi.org/10.1093/eurheartj/ehz486
http://www.ncbi.nlm.nih.gov/pubmed/31497854?tool=bestpractice.com
It is part of a group of drugs called thiazolidinediones. It has a high glucose-lowering effect and does not cause hypoglycaemia.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
It has shown potential cardiovascular benefits, as well as potential benefits in MASLD (also known as NAFLD) and MASH (also known as NASH).[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
The ADA recommends pioglitazone as a preferred agent for hyperglycaemia treatment in people with type 2 diabetes with biopsy-confirmed MASH or at high risk with clinically significant liver fibrosis on non-invasive testing.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
However, it has been associated with fluid retention, congestive heart failure, weight gain, and bone fracture.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
[164]Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020 Aug 18;173(4):278-86.
https://www.acpjournals.org/doi/10.7326/M20-0864?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
http://www.ncbi.nlm.nih.gov/pubmed/32598218?tool=bestpractice.com
The ESC does not recommend use of pioglitazone in patients at risk of or with a history of heart failure due to its association with an increased risk of incident heart failure in patients with diabetes.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Use of pioglitazone is associated with a small increased risk of bladder cancer.[234]US Food and Drug Administration. FDA drug safety communication: updated FDA review concludes that use of type 2 diabetes medicine pioglitazone may be linked to an increased risk of bladder cancer. December 2016 [internet publication].
https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-updated-fda-review-concludes-use-type-2-diabetes-medicine-pioglitazone
Another thiazolidinedione, rosiglitazone, has been removed from the market due to persistent safety concerns.[235]European Medicines Agency. Questions and answers on the suspension of rosiglitazone-containing medicines (Avandia, Avandamet and Avaglim). September 2010 [internet publication].
https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-suspension-rosiglitazone-containing-medicines-avandia-avandamet-avaglim_en.pdf
Antihyperglycaemic pharmacotherapy: insulin-based treatments
When starting insulin therapy, the patient should continue metformin as long as there are no contraindications or intolerances.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Review the continued need for other blood glucose-lowering therapies (e.g., consider additive hypoglycaemia risk and if there are additional benefits of continuing other drugs, such as cardiometabolic or kidney-related).[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Choose the most appropriate insulin type for the patient, and develop an individualised regimen, based on the following options:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Give basal isophane (neutral protamine Hagedorn, NPH) insulin injected once or twice daily according to need
Consider starting both NPH and short-acting insulin (particularly if the person's HbA1c is 75 mmol/mol [9.0%] or higher), administered either:
Consider using insulin detemir or insulin glargine as an alternative to NPH insulin if:
The patient needs assistance from a carer or healthcare professional to inject insulin, and use of insulin detemir or insulin glargine would reduce the frequency of injections from twice to once daily, or
The patient’s lifestyle is restricted by recurrent symptomatic hypoglycaemic episodes, or
The patient would otherwise need twice-daily NPH insulin injections in combination with oral glucose-lowering drugs
Consider pre-mixed (biphasic) preparations that include short-acting insulin analogues, rather than pre-mixed (biphasic) preparations that include short-acting human insulin preparations, if:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
The patient prefers injecting insulin immediately before a meal, or
Hypoglycaemia is a problem, or
Blood glucose levels rise markedly after meals.
Consider switching to insulin detemir or insulin glargine from NPH insulin if the patient:[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Does not reach their target HbA1c because of significant hypoglycaemia, or
Experiences significant hypoglycaemia on NPH insulin irrespective of the level of HbA1c reached, or
Cannot use the device needed to inject NPH insulin but could administer their own insulin safely and accurately if switched to one of the long-acting insulin analogues, or
Needs help from a carer or healthcare professional to administer insulin injections and for whom switching to one of the long-acting insulin analogues would reduce the number of daily injections.
If the patient is on a basal insulin regimen (NPH insulin, insulin detemir, or insulin glargine), monitor for the need for short-acting insulin before meals (or a pre-mixed [biphasic] insulin preparation).[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
If the patient is on pre-mixed (biphasic) insulin, monitor for the need for a further injection of short-acting insulin before meals or for a change to a basal-bolus regimen with NPH insulin or insulin detemir or insulin glargine, if blood glucose control remains inadequate.[36]National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. Jun 2022 [internet publication].
https://www.nice.org.uk/guidance/ng28
Most people with type 2 diabetes will use insulin delivery devices (insulin pens).
These can be adjusted to administer set doses of insulin, are widely available, and offer convenience and accuracy in insulin dosing.
Less frequently, insulin pumps and patch pump systems are used on a case-by-case basis in people who need multiple daily dose insulin.
Insulin pumps are typically reserved for people with type 1 diabetes. If an insulin pump is appropriate for the patient, its use will require significant patient engagement to achieve clinical benefits beyond multiple daily dose injection-based therapy.
Exogenous insulin is a very effective way to lower serum glucose and lower HbA1c, but its use must be guided in most patients by regular self-monitored blood glucose testing (finger stick blood glucose testing) or continuous glucose monitoring.
Hypoglycaemia (glucose ≤3.9 mmol/L [≤70 mg/dL]) is the most serious potential complication of insulin therapy. People who drive need to be particularly careful to avoid hypoglycaemia and should be warned of the dangers. See the Complications section of this topic.
Another significant side effect is weight gain.
Less common side effects may include hunger, nausea, diaphoresis, injection site irritation, or anaphylaxis.
Correction doses of insulin
When basal-bolus insulin is used by motivated and knowledgeable patients, the dose of rapid-acting insulin that is administered before each meal can be based on anticipated carbohydrate content of the upcoming meal and sometimes adjusted for anticipated physical activity.
A correction (or adjustment) dose may be added to the bolus insulin based on the pre-meal blood glucose level. In practice, a conservative approach to calculating a correction dose is to assume 1 unit of insulin will lower the patient’s blood glucose by 2-4 mmol/L (36-72 mg/dL). Correction dosing can also be calculated using the patient's total daily dose of insulin if food intake is stable. The correction dose can be added to the patient's mealtime insulin requirement (whether based on general meal size or carbohydrate counting) and given as the total bolus dose.
Alternatives to a basal-bolus insulin regimen
While NICE recommends consideration of a basal-bolus insulin regimen for patients who are unable to maintain glycaemic targets on basal insulin alone, the ADA/EASD also notes an alternative option of adding a GLP-1 receptor agonist to basal insulin to intensify treatment.[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
Further, the ADA 2024 diabetes guidelines include that, if insulin is used in an adult with type 2 diabetes, it is recommended to use it in combination with a GLP-1 receptor agonist or a dual GIP/GLP-1 receptor agonist for greater glycaemic efficacy (and added weight and hypoglycaemia risk benefits), with the insulin dose adjusted as needed when one of these agents is added or uptitrated.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
Trial data support the combination of basal insulin and a GLP-1 receptor agonist to lower HbA1c and limit weight gain and hypoglycaemia when compared with an intensified insulin regimen.[236]Wysham CH, Lin J, Kuritzky L. Safety and efficacy of a glucagon-like peptide-1 receptor agonist added to basal insulin therapy versus basal insulin with or without a rapid-acting insulin in patients with type 2 diabetes: results of a meta-analysis. Postgrad Med. 2017 May;129(4):436-45.
http://www.ncbi.nlm.nih.gov/pubmed/28294702?tool=bestpractice.com
[237]Maiorino MI, Chiodini P, Bellastella G, et al. Insulin and glucagon-like peptide 1 receptor agonist combination therapy in type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2017 Apr;40(4):614-24.
https://care.diabetesjournals.org/content/40/4/614.long
http://www.ncbi.nlm.nih.gov/pubmed/28325801?tool=bestpractice.com
[238]Aroda VR, Arulandu JR, Cannon AJ. Insulin/glucagon-like peptide-1 receptor agonist combination therapy for the treatment of type 2 diabetes: are two agents better than one? Clin Diabetes. 2018 Apr;36(2):138-47.
https://diabetesjournals.org/clinical/article/36/2/138/32834/Insulin-Glucagon-Like-Peptide-1-Receptor-Agonist
http://www.ncbi.nlm.nih.gov/pubmed/29686453?tool=bestpractice.com
This could be particularly useful for patients with obesity who require high doses of insulin because of insulin resistance, making the side effect of weight gain from insulin use especially problematic.
Evidence shows that an SGLT2 inhibitor can be added to basal insulin to lower blood glucose without any weight gain or hypoglycaemia.[239]Tang H, Cui W, Li D, et al. Sodium-glucose co-transporter 2 inhibitors in addition to insulin therapy for management of type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017 Jan;19(1):142-7.
http://www.ncbi.nlm.nih.gov/pubmed/27598833?tool=bestpractice.com
[240]Rosenstock J, Jelaska A, Zeller C, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015 Oct;17(10):936-48.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5034797
http://www.ncbi.nlm.nih.gov/pubmed/26040302?tool=bestpractice.com
[241]Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014 Jul;37(7):1815-23.
https://care.diabetesjournals.org/content/37/7/1815.long
http://www.ncbi.nlm.nih.gov/pubmed/24929430?tool=bestpractice.com
Such regimens should only be initiated by a diabetes specialist.
Sick-day rules
Ensure the patient is aware that any intercurrent illness can cause glucose levels to rise.[242]Down S. How to advise on sick day rules. Diabetes Primary Care. 2020 Apr;22(3):47-8.
https://diabetesonthenet.com/wp-content/uploads/pdf/dotn024ae8fb1b78500b7bc752b98e9b6d92.pdf
Give the patient clear and individualised oral and written advice (‘sick-day rules’) about how to adapt management during intercurrent illness. Some drugs need to be suspended during intercurrent illness; it is important to ensure the patient is aware that they will need to restart any suspended medication once they are feeling better and able to eat and drink.
DiabetesontheNet: sick day rules
Opens in new window
The SADMANS mnemonic can be helpful as a reminder of drugs to temporarily pause during sick days, where the illness leads to dehydration:[243]Diabetes Canada. Clinical practice guidelines for the prevention and management of diabetes in Canada: appendix 8 - sick-day medication list. 2018 [internet publication].
https://guidelines.diabetes.ca/cpg
S - sulfonylureas
A - ACE inhibitors
D - diuretics, direct renin inhibitors
M - metformin
A - angiotensin-II receptor antagonists
N - non-steroidal anti-inflammatory drugs
S - SGLT2 inhibitors
If an adult with type 2 diabetes is unwell, consider the need to arrange hospital admission or seek specialist advice. Use your clinical judgement, and take into account the patient’s age, frailty, comorbidities, and risk of complications, and the presence of hyperglycaemia, hypoglycaemia, and/or ketosis.
Bear in mind that there is a considerable risk of hypoglycaemia in some patients. This is a particular concern in patients who are older and/or frail, those with reduced appetite (and subsequent reduced oral intake) owing to acute illness, and those taking medications that put them at specific risk of hypoglycaemia (e.g., sulfonylureas and insulin). These patients will need close blood glucose monitoring and may require medication adjustments.
Bariatric surgery for treatment of diabetes in patients with obesity
The World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases guidelines on obesity note that bariatric surgery is the most effective long-term treatment for obesity and many of its associated health conditions.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
Sleeve gastrectomy and Roux-en-Y gastric bypass are currently the most commonly performed bariatric procedures worldwide.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
A comprehensive medical, psychological, and nutritional evaluation involving a multi-disciplinary team should be completed before bariatric surgery is considered, to determine patient suitability and identify any issues that need addressing; lifelong follow-up, as well as cessation of tobacco, alcohol, and drugs, is also required.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
Guidelines from NICE recommend:[117]National Institute for Health and Care Excellence. Obesity: identification, assessment and management. July 2023 [internet publication].
https://www.nice.org.uk/guidance/cg189
Offer an expedited assessment for bariatric surgery to anyone with a BMI of 35 kg/m² or over who has recent-onset type 2 diabetes (diagnosis within last 10 years) as long as they are also receiving, or will receive, assessment by a specialist weight-management team.
Consider an expedited assessment for bariatric surgery for anyone with a BMI of 30 to 34.9 kg/m² who has recent-onset type 2 diabetes as long as they are also receiving, or will receive, assessment in a specialist weight-management team.
Consider an expedited assessment for bariatric surgery for anyone of South Asian, Chinese, other Asian, Middle Eastern, black African, or African-Caribbean family background, who has recent-onset type 2 diabetes using a lower BMI threshold (reduced by 2.5 kg/m²) than other populations.
The 2022 ADA/EASD consensus guideline recommends bariatric surgery should be considered as an option for adults with type 2 diabetes who are appropriate surgical candidates who have:[102]Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2022 Nov 1;45(11):2753-86.
https://diabetesjournals.org/care/article/45/11/2753/147671/Management-of-Hyperglycemia-in-Type-2-Diabetes
http://www.ncbi.nlm.nih.gov/pubmed/36148880?tool=bestpractice.com
A BMI ≥40.0 kg/m² if of non-Asian ancestry
A BMI ≥37.5 kg/m² if of Asian ancestry
A BMI of 35.0 to 39.9 kg/m² (32.5 to 37.4 kg/m² if of Asian ancestry) and who cannot achieve durable weight loss and improvement in comorbidities (including hyperglycaemia) with non-surgical methods.
Further, the ESC recommends that bariatric surgery should be considered for patients at high or very high CVD risk with BMI ≥35 kg/m² when repetitive and structured efforts of lifestyle changes combined with weight-reducing medicines do not result in maintained weight loss.[80]Marx N, Federici M, Schütt K, et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023 Oct 14;44(39):4043-140.
https://academic.oup.com/eurheartj/article/44/39/4043/7238227?login=false
http://www.ncbi.nlm.nih.gov/pubmed/37622663?tool=bestpractice.com
Randomised clinical trials have shown a benefit from bariatric surgery (also referred to as metabolic surgery) compared with medical therapy alone with regard to diabetes remission, glycaemic control, need for glucose-lowering medications, quality of life, and reduction in cardiovascular risk factor markers over the short term (e.g., 1-3 years) in people with type 2 diabetes, as well as for possible prevention of type 2 diabetes.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
[244]Carlsson LM, Peltonen M, Ahlin S, et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012 Aug 23;367(8):695-704.
https://www.nejm.org/doi/10.1056/NEJMoa1112082?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/22913680?tool=bestpractice.com
[245]Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012 Apr 26;366(17):1567-76.
https://www.nejm.org/doi/10.1056/NEJMoa1200225?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dwww.ncbi.nlm.nih.gov
http://www.ncbi.nlm.nih.gov/pubmed/22449319?tool=bestpractice.com
[246]Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017 Feb 16;376(7):641-51.
https://www.nejm.org/doi/10.1056/NEJMoa1600869
http://www.ncbi.nlm.nih.gov/pubmed/28199805?tool=bestpractice.com
[247]Kashyap SR, Bhatt DL, Wolski K, et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care. 2013 Aug;36(8):2175-82.
http://care.diabetesjournals.org/content/36/8/2175.long
http://www.ncbi.nlm.nih.gov/pubmed/23439632?tool=bestpractice.com
[248]Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013 Jun 5;309(21):2240-9.
http://jamanetwork.com/journals/jama/fullarticle/1693889
http://www.ncbi.nlm.nih.gov/pubmed/23736733?tool=bestpractice.com
[249]Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014 Jul;149(7):716-26.
http://jamanetwork.com/journals/jamasurgery/fullarticle/1876617
http://www.ncbi.nlm.nih.gov/pubmed/24899464?tool=bestpractice.com
Cohort studies suggest that both Roux en Y bypass and sleeve gastrectomy procedures lead to diabetes remission that lasts a mean of about 5 years in more than half of patients, and significantly reduce mortality, stroke, myocardial infarction, and microvascular complications in those with type 2 diabetes.[250]Yska JP, van Roon EN, de Boer A, et al. Remission of type 2 diabetes mellitus in patients after different types of bariatric surgery: a population-based cohort study in the United Kingdom. JAMA Surg. 2015 Dec;150(12):1126-33.
https://jamanetwork.com/journals/jamasurgery/fullarticle/2446843
http://www.ncbi.nlm.nih.gov/pubmed/26422580?tool=bestpractice.com
[251]Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018 Oct 16;320(15):1570-82.
https://jamanetwork.com/journals/jama/fullarticle/2707461
http://www.ncbi.nlm.nih.gov/pubmed/30326126?tool=bestpractice.com
[252]O'Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med. 2018 Sep 4;169(5):300-10.
http://www.ncbi.nlm.nih.gov/pubmed/30083761?tool=bestpractice.com
A systematic review and meta-analysis of randomised-controlled trials concluded that Roux-en-Y bypass resulted in a higher rate of type 2 diabetes remission compared with sleeve gastrectomy after 1 year, but that remission rates did not differ in studies with a 2- and 5-year follow-up.[253]Borgeraas H, Hofsø D, Hertel JK, et al. Comparison of the effect of Roux-en-Y gastric bypass and sleeve gastrectomy on remission of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2020 Jun;21(6):e13011.
https://onlinelibrary.wiley.com/doi/10.1111/obr.13011
http://www.ncbi.nlm.nih.gov/pubmed/32162437?tool=bestpractice.com
Compared with sleeve gastrectomy, Roux en Y leads to somewhat greater weight loss and other benefits, but is a more technically challenging operation with higher re-operation and readmission rates.
The benefits and risks of bariatric surgery also vary substantially across type 2 diabetes patient subgroups. In observational studies, average benefits appear to be highest in people with more recent onset of type 2 diabetes, and those not on insulin therapy.[254]O'Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010 Sep;20(9):1199-205.
http://www.ncbi.nlm.nih.gov/pubmed/20532834?tool=bestpractice.com
[255]Park JY. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr. 2018 Dec 30;27(4):213-22.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6513303
http://www.ncbi.nlm.nih.gov/pubmed/31089566?tool=bestpractice.com
Benefits have been documented in younger people (age 40-50 years) as well as those over 65 years of age.[254]O'Keefe KL, Kemmeter PR, Kemmeter KD. Bariatric surgery outcomes in patients aged 65 years and older at an American Society for Metabolic and Bariatric Surgery Center of Excellence. Obes Surg. 2010 Sep;20(9):1199-205.
http://www.ncbi.nlm.nih.gov/pubmed/20532834?tool=bestpractice.com
[255]Park JY. Prediction of type 2 diabetes remission after bariatric or metabolic surgery. J Obes Metab Syndr. 2018 Dec 30;27(4):213-22.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6513303
http://www.ncbi.nlm.nih.gov/pubmed/31089566?tool=bestpractice.com
Bariatric surgery procedures, in particular Roux-en-Y gastric bypass, have been associated with subsequent alcohol-related complications.[28]World Gastroenterology Organisation and International Federation for the Surgery of Obesity and Metabolic Diseases. IFSO-WGO guidelines on obesity. 2023 [internet publication].
https://www.worldgastroenterology.org/guidelines/obesity/obesity-english
[256]Mahmud N, Panchal S, Abu-Gazala S, et al. Association between bariatric surgery and alcohol use-related hospitalization and all-cause mortality in a veterans affairs cohort. JAMA Surg. 2023 Feb 1;158(2):162-71.
https://pubmed.ncbi.nlm.nih.gov/36515960
http://www.ncbi.nlm.nih.gov/pubmed/36515960?tool=bestpractice.com
It is important to take this into account when selecting patients for these procedures, and to consider alcohol-related counselling.[256]Mahmud N, Panchal S, Abu-Gazala S, et al. Association between bariatric surgery and alcohol use-related hospitalization and all-cause mortality in a veterans affairs cohort. JAMA Surg. 2023 Feb 1;158(2):162-71.
https://pubmed.ncbi.nlm.nih.gov/36515960
http://www.ncbi.nlm.nih.gov/pubmed/36515960?tool=bestpractice.com
Planning pregnancy
Women with type 2 diabetes should use an effective method of contraception until they plan pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Women should be evaluated before pregnancy for retinopathy, nephropathy, neuropathy, and possible cardiovascular disease, which may worsen during or complicate pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Explain to women with diabetes who are planning pregnancy that:[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
If they have good blood glucose control before conception and throughout their pregnancy, this will reduce the risk of miscarriage, congenital malformation, stillbirth, and neonatal death
The risks can be reduced but not eliminated.
Agree individualised targets for self-monitoring of blood glucose with women who have diabetes and are planning a pregnancy, taking into account the risk of hypoglycaemia.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
NICE recommends that HbA1c should be <48 mmol/mol (6.5%) before conception if this can be achieved without problematic hypoglycaemia.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Any reduction towards this target is likely to reduce the risk of congenital malformations. NICE recommends up to monthly measurement of HbA1c levels for women with diabetes who are planning a pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Strongly advise women with diabetes whose HbA1c level is above 86 mmol/mol (10%) not to get pregnant until their HbA1c level is lower, because of the associated risks.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Review the patient’s medication. Stop any drugs contraindicated in pregnancy if your patient is planning pregnancy or as soon as pregnancy is confirmed; use alternative agents that are suitable for pregnant women.
Women with diabetes may be advised to use metformin (with or without insulin) in the preconception period (and during pregnancy), when the likely benefits from improved blood glucose control outweigh the potential for harm. Stop all other oral blood glucose-lowering agents before (and throughout) pregnancy, including SGLT2 inhibitors.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
[136]Herrington WG, Frankel AH. UK Kidney Association clinical practice guideline: sodium-glucose co-transporter-2 (SGLT-2) inhibition in adults with kidney disease. Apr 2023 [internet publication].
https://guidelines.ukkidney.org
Stop ACE inhibitors and angiotensin-II receptor antagonists before conception, or as soon as pregnancy is confirmed.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Stop statins before pregnancy, or as soon as pregnancy is confirmed.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Advise women who are planning a pregnancy to take folic acid (5 mg/day), which should be continued until 12 weeks of gestation.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Women with diabetes have an increased risk of having infants with neural tube defects, compared with the general population.[257]Tinker SC, Gilboa SM, Moore CA, et al. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997-2011. Am J Obstet Gynecol. 2020 Feb;222(2):176.e1-11.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7186569
http://www.ncbi.nlm.nih.gov/pubmed/31454511?tool=bestpractice.com
During pregnancy
During pregnancy, women should be cared for by a multi-disciplinary team, including a dietitian, a nurse educator, an endocrinologist, and an obstetrician.
Offer pregnant women with pre-existing diabetes retinal assessment by digital imaging with mydriasis using tropicamide following their first antenatal clinic appointment (unless they have had a retinal assessment in the last 3 months), and again at 28 weeks. If any diabetic retinopathy is present at booking, perform an additional retinal assessment at 16-20 weeks.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
NICE guidelines recommend the following blood glucose targets in pregnant women with pre-existing type 2 diabetes (as long as these are achievable without causing problematic hypoglycaemia):[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
[Evidence C]4efe3e6f-336e-471b-b4bd-bb6de160be6aguidelineCWhat are the effects of tighter blood glucose control compared with less tight blood glucose control in pregnant women with gestational diabetes?[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Fasting: <5.3 mmol/L (<95.4 mg/dL), and
1 hour after meals: <7.8 mmol/L (<140.4 mg/dL), or
2 hours after meals: <6.4 mmol/L (<115.2 mg/dL).
Advise pregnant women with diabetes who are on insulin to maintain their capillary plasma glucose level above 4 mmol/L (72 mg/dL).[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Measure HbA1c levels in all pregnant women with pre-existing diabetes at the booking appointment to determine the level of risk for the pregnancy. Consider measuring HbA1c levels in the second and third trimesters of pregnancy for women with pre-existing diabetes to assess the level of risk for the pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Review the patient’s medication. Stop any agents contraindicated in pregnancy as soon as pregnancy is confirmed and use alternatives that are suitable for pregnant women, as described in the 'Planning pregnancy' section above.
Women who are breastfeeding can resume or continue metformin immediately after birth, but should avoid other oral blood glucose-lowering therapy while breastfeeding.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Women with diabetes who are breastfeeding should continue to avoid any medicines for their diabetes complications that were stopped for safety reasons when they started planning the pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
NICE recommends NPH insulin as the first choice for long-acting insulin during pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Consider continuing treatment with long-acting insulin analogues (insulin detemir or insulin glargine) for women with diabetes who have established good blood glucose control while using these before pregnancy.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
The available evidence on rapid-acting insulin analogues (insulin aspart and insulin lispro) does not show an adverse effect on pregnancy or the health of the baby.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
In practice, the majority of pregnant women with type 2 diabetes will need insulin.
Pregnant women should test their fasting, pre-meal, 1-hour post-meal, and bedtime blood glucose levels every day.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
The pattern should be examined every few weeks early in pregnancy so that nutrition content and timing, exercise patterns, and the insulin doses can be modified to achieve optimal control. CGM monitoring during pregnancy may be considered on an individual basis (in addition to self-monitoring) for those taking insulin who are experiencing problematic severe hypoglycaemia or who have unstable blood glucose levels causing concern despite efforts to optimise control.[34]American Diabetes Association. Standards of care in diabetes - 2024. Diabetes Care. 2024 Jan;47(suppl 1):S1-321.
https://diabetesjournals.org/care/issue/47/Supplement_1
[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
Insulin requirements generally increase early in pregnancy, then decrease from about 8 to 16 weeks before rising throughout the rest of the pregnancy.
Advise pregnant women with type 2 diabetes to take low-dose aspirin from 12 weeks until the birth of the baby.[99]National Institute for Health and Care Excellence. Diabetes in pregnancy: management from preconception to the postnatal period. Dec 2020 [internet publication].
https://www.nice.org.uk/guidance/ng3
[258]National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. April 2023 [internet publication].
https://www.nice.org.uk/guidance/ng133

