Aetiology
Lyme disease is caused by spirochetes belonging to the Borrelia burgdorferi sensu lato (sl) complex, which consists of around 20 genospecies with a complex genomic structure. Not all B burgdorferi sl species are pathogenic.[1] Lyme disease in North America is mainly caused by Borrelia burgdorferi sensu stricto and is transmitted by the ticks Ixodes scapularis and I pacificus. A new pathogenic genospecies of B burgdorferi sl, B mayonii, has been described in the upper midwestern US. This pathogen was isolated from, and genetically identified in, both humans and I scapularis ticks. B mayonii causes Lyme borreliosis with unusually high spirochetaemia.[15] Lyme disease in Europe and Asia is predominantly caused by B afzelii and B garinii.[1]
Borrelia species are spirochetes (motile, spiral-shaped bacteria) that have 7 to 11 flagella and an outer membrane containing an abundance of outer surface proteins (Osp). These proteins, designated by letters A through F, are encoded by plasmids. OspA and OspC are potentially useful for developing vaccines against Lyme disease.[16]
The major reservoirs for B burgdorferi are mice, voles, squirrels, birds, and other small animals. Deer are not a major reservoir, but they are a major host for adult Ixodes ticks, the vectors of transmission. These ticks are largely confined to temperate climate zones of the northern hemisphere.[1]
I scapularis is the primary vector in the northeastern and midwestern US. The nymphal stage, which is prevalent in spring and early summer, is most likely to transmit the infection through its bite, although infection can be acquired at all 3 stages (larval, nymphal, adult).
I ricinus is the principal vector in Europe, and I persulcatus is the principal vector in large parts of Russia and Asia.[1] On the western coast of the US, I pacificus serves as the predominant vector.[17] In Europe, 12% of nymphal and 15% of adult I ricinus ticks are infected with B burgdorferi sl, and 2% to 3% of humans develop Lyme disease after a tick bite. Incidence is similar in the US.[1]
[Figure caption and citation for the preceding image starts]: Deer tick (or black-legged tick), Ixodes scapularis, as it was questing on a blade of grassCDC Image Library [Citation ends]. [Figure caption and citation for the preceding image starts]: Under a high magnification, this digitally-colourised scanning electron micrograph depicts three gram-negative, anaerobic, Borrelia burgdorferi bacteria, which had been derived from a pure cultureCDC Image Library [Citation ends].
[Figure caption and citation for the preceding image starts]: Under a high magnification, this digitally-colourised scanning electron micrograph depicts three gram-negative, anaerobic, Borrelia burgdorferi bacteria, which had been derived from a pure cultureCDC Image Library [Citation ends].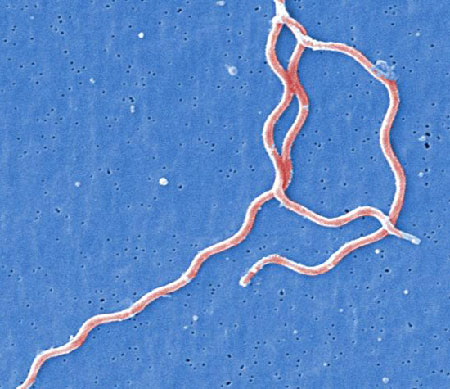
Pathophysiology
B burgdorferi is inoculated into the skin by the feeding Ixodes tick, usually after the tick has fed for more than 48 hours. Initial infection is established at the tick bite site. After skin inoculation, B burgdorferi moves throughout the extracellular matrix by binding to components such as epithelial cell-derived proteoglycans and interacting with decorin, glycosaminoglycans, and fibronectin. This leads to expansion of the rash.[18]Borrelia disseminates from skin to other organs quickly. It replicates, kills host cells, and emerges through the membrane of these cells. Within days to weeks after infection, Borrelia has been recovered from blood, cerebrospinal fluid, myocardium, retina, muscle, bone, spleen, liver, meninges, and brain.[19]
The host immune response to Borrelia integrates both cell-mediated and humoral mechanisms. Most patients have an IgM antibody response against OspC or the flagellar protein (41-kDa) of Borrelia within days of days onset. Humoral immune responses may be initially limited, and a clinically detectable level of antibodies may be delayed. Early antibiotic treatment may delay or abrogate the B-lymphocytic response. In the chronic phase, antibodies against a variety of Borrelia epitopes become detectable. Although the B-cell response in the chronic phase is robust, it is not preventive of future infection(s).[18]
Persistent clinical symptoms, such as those of chronic joint inflammation, have been attributed to autoimmunity. Activated T lymphocytes and lymphokines of the helper T-cell phenotype (CD4 cells) play a major role in the pathogenesis of Lyme arthritis.[18]
Use of this content is subject to our disclaimer