Primary prevention
Active immunisation with tetanus vaccine protects against tetanus.[26] In most cases, 5 intramuscular doses at appropriate intervals give lifelong immunity. The vaccine is a purified toxin extracted from a strain of Clostridium tetani, which is treated with formaldehyde to produce tetanus toxoid.[4] International immunisation recommendations and schedules may vary, and local guidelines should be consulted.
UK schedule for tetanus immunisation:[4] UK Health Security Agency: complete routine immunisation schedule Opens in new window
Primary immunisation in infants and children aged <10 years: DTaP (D=diphtheria, T=tetanus, aP=acellular pertussis) given in combination with inactivated polio vaccine (IPV)/Haemophilus influenzae type b (Hib)/hepatitis B at ages 2, 3, and 4 months.
Primary immunisation in children aged >10 years and adults: 3 doses of Td (T=tetanus, d=low-dose diphtheria) given in combination with IPV, with an interval of 1 month between each dose.
First booster in children aged <10 years: DTaP (given in combination with IPV) ideally given at least 3 years after completion of the primary immunisation course.
First booster in people aged >10 years: Td (given in combination with IPV) for those who have undergone primary immunisation, with the last dose 5 years or more ago.
Second booster, all patients: Td (given in combination with IPV), ideally given 10 years after the first booster.
Travellers
Travellers at greatest risk for exposure and infection include humanitarian aid workers, pregnant travellers, and travellers not current with tetanus toxoid-containing vaccine. It is particularly important that those travelling to remote areas check they are up to date with tetanus vaccination before departure as tetanus immunoglobulin (TIG) and proper wound management may not be available, should a tetanus-prone injury occur.[27]
People who inject drugs
People who inject drugs should be fully immunised. Drug practices that are less tetanus prone can be encouraged, such as avoiding intramuscular and subcutaneous injection and using as little citric acid as possible, which devitalises tissue.[23]
Pregnant women
Immunisation of pregnant women, or women of childbearing age, with at least 2 doses of tetanus toxoid is estimated to decrease mortality from neonatal tetanus by 94%.[28] The American College of Obstetricians and Gynecologists has made the following recommendations regarding immunisation during pregnancy.[29]
The tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap [T=tetanus, d=low-dose diphtheria, ap=acellular pertussis]) vaccine should be administered during each pregnancy, as early in the 27 to 36 weeks’ gestation window as possible.
In extenuating circumstances, it may be appropriate for a pregnant woman to receive the Tdap vaccine outside of this window, for example in cases of wound management or a pertussis outbreak.
If the Tdap vaccine is not administered during pregnancy, it should be given immediately postnatal if the woman has never received a prior dose of Tdap as an adolescent, adult, or during a previous pregnancy.
If the Tdap vaccine is administered early in the woman’s pregnancy (i.e., before 27 to 36 weeks of gestation), the woman does not need to be vaccinated again during 27 to 36 weeks of gestation.
Neonatal tetanus
In resource-poor settings, the World Health Organization advocates six clean methods to improve birth hygiene: clean birth surface, clean hands, clean perineum, cord cutting, cord tying, and cord care.[16][30]
Available evidence supports the implementation of immunisation practices for women of childbearing age or pregnant women in communities with high levels of risk of neonatal tetanus.[31] [
 ]
]
US schedule for tetanus immunisation[32][33][34]
Primary immunisation in infants and children aged 0 to 6 years: 5-dose series of DTaP given at 2, 4, 6, and 15 to 18 months of age, and 4 to 6 years of age.
First booster in adolescents aged 11 to 12 years: 1 dose of Tdap.
Second booster, all patients: 1 dose of Td or Tdap should be given every 10 years.
Patients who did not previously receive Tdap at or after age 11 years should receive one dose of Tdap, then Td or Tdap every 10 years. Adults who have not previously received the primary vaccination series should be given one dose of Tdap, followed by one dose of Td or Tdap at least 4 weeks later, and then another dose of Td or Tdap 6 to 12 months after the second dose. Td or Tdap booster doses are then given every 10 years.
A hexavalent vaccine is also approved by the US Food and Drug Administration to prevent diphtheria, tetanus, pertussis, polio, Haemophilus influenzae b, and hepatitis B. The DTaP-IPV-Hib-HepB vaccine is licensed for use in children aged 6 weeks to 4 years and is indicated for the primary vaccination series in infants at ages 2, 4, and 6 months.[35]
Pregnant women should be vaccinated with Tdap during each pregnancy, ideally between 27 and 36 weeks' gestation.
Tdap may be administered regardless of the time interval since the most recent tetanus-containing or diphtheria-toxoid-containing vaccine.
Management of tetanus-prone wounds
Management of tetanus-prone wounds to prevent clinical tetanus depends on risk assessment of the wound and the immunisation history of the patient.
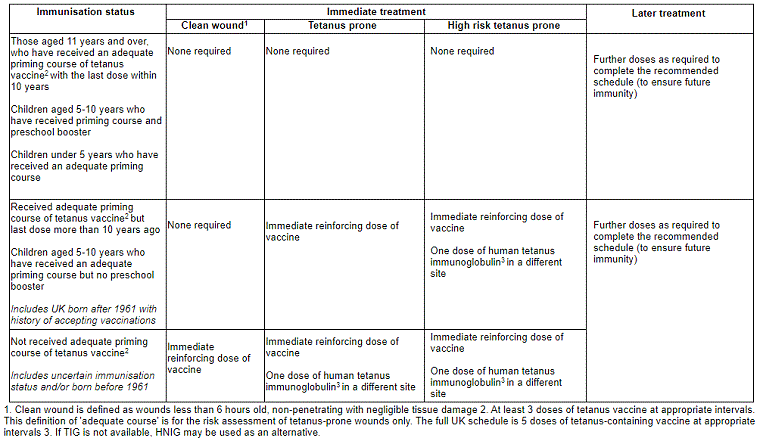
In the UK, management of tetanus-prone wounds to prevent clinical tetanus depends on risk assessment of the wound and the immunisation history of the patient. See table below. No immediate treatment is needed in some scenarios, or the patient may require an immediate reinforcing dose of vaccine with or without a dose of human TIG.[4][37] Clean wounds do not require human TIG.[3][4][36][Figure caption and citation for the preceding image starts]: Immunisation recommendations for clean and tetanus-prone woundsUK Health Security Agency. Tetanus: the green book, chapter 30. June 2022 [Citation ends].
In the US, patients with clean, minor wounds who have only had up to 2 doses of tetanus toxoid-containing vaccine or an uncertain vaccination history should be given tetanus toxoid-containing vaccine, while patients who have received ≥3 doses do not require tetanus toxoid-containing vaccine unless they have not received a dose in the last 10 years. Clean, minor wounds do not require TIG.[3][36] For all other wounds, patients who have only had up to 2 doses of tetanus toxoid-containing vaccine or have an uncertain vaccination history should be given tetanus toxoid-containing vaccine and TIG; patients who have received ≥3 doses do not require TIG and do not require tetanus toxoid-containing vaccine unless they have not received a dose in the last 5 years.[3][36] [Figure caption and citation for the preceding image starts]: US recommendations for tetanus wound management. DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis; Td = tetanus and diphtheria toxoids; TIG = tetanus immunoglobulin. *DTaP is recommended for children aged <7 years. Tdap is preferred to Td for persons aged ≥11 years who have not previously received Tdap. Persons aged ≥7 years who are not fully immunised against pertussis, tetanus or diphtheria should receive one dose of Tdap for wound management and as part of the catch-up series. **Immunosuppressed patients should be managed as if they were incompletely immunised (i.e., those with contaminated wounds should also receive TIG, regardless of their history of tetanus immunisation)Liang JL et al. Prevention of pertussis, tetanus, and diphtheria with vaccines in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2018;67:1-44. [Citation ends].
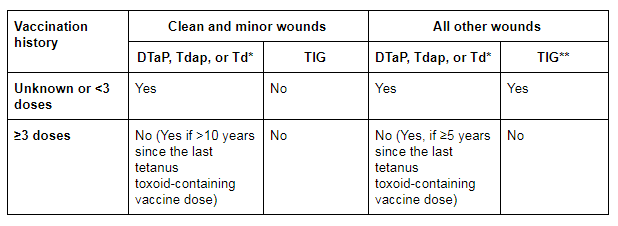
Immunosuppressed patients may not be adequately protected and additional boosting and/or TIG may be required; in the US, immunosuppressed patients should be managed as if they were incompletely immunised.[3][4][36]
In the UK, the dose of human TIG for prevention in a patient with a tetanus-prone wound is 250 international units by intramuscular injection, or 500 international units if more than 24 hours has elapsed since injury, or if heavy contamination is possible or following burns. If intramuscular TIG cannot be sourced, UK guidelines, recommend that human normal immunoglobulin for subcutaneous or intramuscular use may be given as an alternative.[4]
If a tetanus booster is indicated for wound management during pregnancy, Tdap should be administered instead of Td if the woman has not received Tdap previously.[29]
UK guidelines for the management of clean and tetanus-prone wounds recommend the use of intramuscular TIG for 'high-risk' tetanus-prone wounds, irrespective of the tetanus immunisation history of the patient. 'High risk' is regarded as heavy contamination with material likely to contain tetanus spores and/or extensive devitalised tissue.[4]
Secondary prevention
Natural disease does not confer immunity; therefore, full tetanus immunisation must be undertaken.
Use of this content is subject to our disclaimer