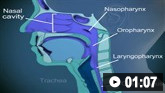
A multidisciplinary approach to the acute phase is required, combining supportive therapy and disease-modifying therapy with either high-dose intravenous immunoglobulin (IVIG) or plasma exchange.[12]Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016 Aug 13;388(10045):717-27.
https://www.doi.org/10.1016/S0140-6736(16)00339-1
http://www.ncbi.nlm.nih.gov/pubmed/26948435?tool=bestpractice.com
IVIG and plasma exchange are equally efficacious.
Supportive therapy: respiratory management
Respiratory failure is common in GBS, and approximately 20% to 30% of patients need ventilatory support in an intensive care unit (ICU).[12]Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016 Aug 13;388(10045):717-27.
https://www.doi.org/10.1016/S0140-6736(16)00339-1
http://www.ncbi.nlm.nih.gov/pubmed/26948435?tool=bestpractice.com
[129]Walgaard C, Lingsma HF, Ruts L, et al. Prediction of respiratory insufficiency in Guillain-Barré syndrome. Ann Neurol. 2010 Jun;67(6):781-7.
http://www.ncbi.nlm.nih.gov/pubmed/20517939?tool=bestpractice.com
Risk factors for progression to mechanical ventilation include: short time from symptom onset to hospital admission, bulbar, neck, or facial weakness, severe muscle weakness at hospital admission, and autonomic instability.[128]Green C, Baker T, Subramaniam A. Predictors of respiratory failure in patients with Guillain-Barré syndrome: a systematic review and meta-analysis. Med J Aust. 2018 Mar 5;208(4):181-8.
http://www.ncbi.nlm.nih.gov/pubmed/29490222?tool=bestpractice.com
[129]Walgaard C, Lingsma HF, Ruts L, et al. Prediction of respiratory insufficiency in Guillain-Barré syndrome. Ann Neurol. 2010 Jun;67(6):781-7.
http://www.ncbi.nlm.nih.gov/pubmed/20517939?tool=bestpractice.com
Algorithms or tools that predict a patient's risk of respiratory failure at admission (e.g., the Erasmus GBS Respiratory Insufficiency Score [EGRIS]) may be more reliable than individual variables.[128]Green C, Baker T, Subramaniam A. Predictors of respiratory failure in patients with Guillain-Barré syndrome: a systematic review and meta-analysis. Med J Aust. 2018 Mar 5;208(4):181-8.
http://www.ncbi.nlm.nih.gov/pubmed/29490222?tool=bestpractice.com
[129]Walgaard C, Lingsma HF, Ruts L, et al. Prediction of respiratory insufficiency in Guillain-Barré syndrome. Ann Neurol. 2010 Jun;67(6):781-7.
http://www.ncbi.nlm.nih.gov/pubmed/20517939?tool=bestpractice.com
[11]Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019 Nov;15(11):671-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638
http://www.ncbi.nlm.nih.gov/pubmed/31541214?tool=bestpractice.com
Pulse oximetry and arterial blood gases should not be relied on, as hypoxia or hypercapnia is a late sign and patients will decompensate very quickly.
There is insufficient evidence to recommend specific methods for monitoring respiratory function, but respiratory status should be monitored in all patients.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
Bedside spirometry, including measurement of forced vital capacity, should be performed every 6 hours initially. Early spirometry will also help triage the patient between the ICU and regular ward.
Patients with bulbar dysfunction, high risk of aspiration (i.e., infiltrates on chest x-ray), and new atelectasis on chest x-ray should be intubated early for airway protection and impending respiratory failure.
In patients with no bulbar dysfunction, or with mild bulbar dysfunction without aspiration risk, the 20/30/40 rule should be used.[125]Lawn ND, Fletcher DD, Henderson RD, et al. Anticipating mechanical ventilation in Guillain-Barré syndrome. Arch Neurol. 2001 Jun;58(6):893-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/779520
http://www.ncbi.nlm.nih.gov/pubmed/11405803?tool=bestpractice.com
The patient should be monitored in the ICU and elective intubation considered if any of the following is present:
Vital capacity is <20 mL/kg
Maximal inspiratory pressure is worse than -30 cmH₂O (negative inspiratory force)
Maximal expiratory pressure is <40 cmH₂O
Vital capacity, maximal inspiratory pressure, or maximal expiratory pressure is reduced by 30% or more from baseline.[125]Lawn ND, Fletcher DD, Henderson RD, et al. Anticipating mechanical ventilation in Guillain-Barré syndrome. Arch Neurol. 2001 Jun;58(6):893-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/779520
http://www.ncbi.nlm.nih.gov/pubmed/11405803?tool=bestpractice.com
The reported mean duration of ventilation is 15 to 43 days, and weaning should be guided by serial pulmonary function tests (PFTs) and assessment of strength.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
The need for tracheostomy should be addressed from week 2 onwards, especially if PFTs do not show improvement. If there is improvement of PFTs above baseline, tracheostomy may be delayed by an additional week before reassessment.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
Supportive therapy: cardiovascular management
Haemodynamic monitoring of pulse and blood pressure (BP) should be started on admission. Telemetry is prudent, especially if there is evidence of dysautonomia. If dysautonomia is present, continuous cardiac monitoring and placement of a Foley catheter should be initiated on admission. There are insufficient data for methods and setting of monitors, but all patients with severe disease should have their pulse and BP monitored until they are off ventilator support and have begun to recover.[17]Ho TW, Mishu B, Li CY, et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995 Jun;118 ( Pt 3):597-605.
http://www.ncbi.nlm.nih.gov/pubmed/7600081?tool=bestpractice.com
[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
Fluid balance should be monitored carefully, because the autonomic dysfunction renders clinical determination of hydration status very difficult. Hypotensive episodes can be managed with fluid boluses.
If BP is very labile, intra-arterial BP monitoring should be initiated. Hypertensive episodes should be treated with short-acting agents (e.g., labetalol, esmolol, nitroprusside) to prevent abrupt hypotension.
Other factors that may potentiate dysautonomia include manoeuvres such as suctioning and changing position (i.e., lying to sitting), and medicines (antihypertensive drugs, succinylcholine).[154]Truax B. Autonomic disturbances in Guillain-Barré syndrome. Semin Neurol. 1984;4(4):462-8.
Supportive therapy: deep vein thrombosis (DVT) prophylaxis
Immobility and hypercoagulability from treatments such as IVIG can increase the risk of DVT in these patients.[155]Lawn ND, Wijdicks EF. Fatal Guillain-Barré syndrome. Neurology. 1999 Feb;52(3):635-8.
http://www.ncbi.nlm.nih.gov/pubmed/10025803?tool=bestpractice.com
Appropriate prophylactic anticoagulation (e.g., a direct oral anticoagulant, subcutaneous unfractionated heparin or a low molecular weight heparin) and support stockings are recommended for non-ambulatory patients until they are able to walk independently.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
Supportive therapy: pain management
Various medications (e.g., gabapentin, carbamazepine, amitriptyline) may be helpful in the acute and long-term management of neuropathic pain associated with GBS.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
Opioids may aggravate autonomic gut dysmotility and bladder distension, and should be used with caution.[156]Zochodne DW. Autonomic involvement in Guillain-Barré syndrome: a review. Muscle Nerve. 1994 Oct;17(10):1145-55.
http://www.ncbi.nlm.nih.gov/pubmed/7935521?tool=bestpractice.com
[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
[157]Burns TM, Lawn ND, Low PA, et al. Adynamic ileus in severe Guillain-Barré syndrome. Muscle Nerve. 2001 Jul;24(7):963-5.
http://www.ncbi.nlm.nih.gov/pubmed/11410925?tool=bestpractice.com
Choice of immunotherapy
Immunotherapy comprises IVIG or plasma exchange.
IVIG and plasma exchange are equally efficacious.[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
[11]Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019 Nov;15(11):671-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638
http://www.ncbi.nlm.nih.gov/pubmed/31541214?tool=bestpractice.com
One Cochrane review found moderate-quality evidence that, in severe disease, IVIG given within 2 weeks of disease onset expedites recovery to a similar extent as plasma exchange.[52]Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014 Sep 19;(9):CD002063.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002063.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/25238327?tool=bestpractice.com
Combination therapy (plasma exchange followed by IVIG) is not recommended.[52]Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014 Sep 19;(9):CD002063.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002063.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/25238327?tool=bestpractice.com
[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
[11]Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019 Nov;15(11):671-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638
http://www.ncbi.nlm.nih.gov/pubmed/31541214?tool=bestpractice.com
IVIG is administered via peripheral intravenous infusion. It is used more frequently than plasma exchange because plasma exchange requires central venous access and is associated with tolerability issues.[159]Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017 Mar;139(3s):S1-46.
https://www.jacionline.org/article/S0091-6749(16)31141-1/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/28041678?tool=bestpractice.com
Treatment-related complications occur less frequently with IVIG than with plasma exchange.[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
IVIG is a pooled blood product and is associated with the risk of pathogen transmission (e.g., HIV, hepatitis B or C, Creutzfeldt-Jakob disease), although the risk is low.
IVIG can precipitate anaphylaxis in an IgA-deficient person, and is contraindicated in these patients. Plasma exchange is preferred in the presence of ongoing renal failure.
Large randomised clinical trials have established the effectiveness of plasma exchange in severe disease.[14]The French Cooperative Group on Plasma Exchange in Guillain-Barré Syndrome. Appropriate number of plasma exchanges in Guillain-Barré syndrome. Ann Neurol. 1997 Mar;41(3):298-306.
http://www.ncbi.nlm.nih.gov/pubmed/9066350?tool=bestpractice.com
[160]French Cooperative Group on Plasma Exchange in Guillain-Barré syndrome. Efficiency of plasma exchange in Guillain-Barré syndrome: role of replacement fluids. Ann Neurol. 1987 Dec;22(6):753-61.
http://www.ncbi.nlm.nih.gov/pubmed/2893583?tool=bestpractice.com
[161]Osterman PO, Fagius J, Lundemo G, et al. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet. 1984 Dec 8;2(8415):1296-9.
http://www.ncbi.nlm.nih.gov/pubmed/6150321?tool=bestpractice.com
Plasma exchange is recommended for ambulatory patients >2 weeks from onset of neurological symptoms because trials with IVIG did not include these patients.[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
[162]Donofrio PD, Berger A, Brannagan TH 3rd, et al. Consensus statement: the use of intravenous immunoglobulin in the treatment of neuromuscular conditions report of the AANEM ad hoc committee. Muscle Nerve. 2009 Nov;40(5):890-900.
http://www.ncbi.nlm.nih.gov/pubmed/19768755?tool=bestpractice.com
Evidence suggests that in adults with GBS, plasma exchange is superior to supportive care with respect to:[50]Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017 Feb 27;(2):CD001798.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001798.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28241090?tool=bestpractice.com
Mean time to recover walking with aid (primary outcome)
Shorter time to onset of recovery (primary outcome)
Improvement by one disability grade by 4 weeks (secondary outcome).
There is no evidence concerning the relative efficacy of plasma exchange and IVIG for treating axonal forms of GBS.
Corticosteroid monotherapy does not significantly shorten time to recovery or prevent long-term disability in patients with GBS; oral corticosteroids delay recovery compared with placebo, possibly due to harmful effects on denervated muscle.[163]Hughes RA, Brassington R, Gunn AA, et al. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2016 Oct 24;(10):CD001446.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001446.pub5/full
http://www.ncbi.nlm.nih.gov/pubmed/27775812?tool=bestpractice.com
[  ]
What are the benefits and harms of corticosteroids in people with Guillain‐Barré syndrome?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.1497/fullShow me the answer[Evidence B]eb2545f6-7bfb-4eda-ae95-1c163e6ad598ccaBWhat are the benefits and harms of corticosteroids in people with Guillain‐Barre syndrome?
]
What are the benefits and harms of corticosteroids in people with Guillain‐Barré syndrome?/cca.html?targetUrl=https://www.cochranelibrary.com/cca/doi/10.1002/cca.1497/fullShow me the answer[Evidence B]eb2545f6-7bfb-4eda-ae95-1c163e6ad598ccaBWhat are the benefits and harms of corticosteroids in people with Guillain‐Barre syndrome?
Intravenous immunoglobulin (IVIG)
IVIG is recommended for:[52]Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2014 Sep 19;(9):CD002063.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002063.pub6/full
http://www.ncbi.nlm.nih.gov/pubmed/25238327?tool=bestpractice.com
[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
One small retrospective study did not find a difference in outcomes for two groups given IVIG either within 7 days of symptom onset or 7 days or more after symptom onset.[164]Guzey Aras Y, Tanik O, Dogan Güngen B, et al. Patient characteristics and the effects of intravenous immunoglobulin in patients with Guillain-Barre syndrome. Ideggyogy Sz. 2016 Nov 30;69(11-12):389-95.
http://www.ncbi.nlm.nih.gov/pubmed/29733556?tool=bestpractice.com
A randomised controlled trial found no evidence of any benefit of a second IVIG course for patients with GBS with a poor prognosis, and there was a risk of serious adverse events. Therefore a second course of IVIG is not recommended.[165]Walgaard C, Jacobs BC, Lingsma HF, et al. Second intravenous immunoglobulin dose in patients with Guillain-Barré syndrome with poor prognosis (SID-GBS): a double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2021 Apr;20(4):275-83.
http://www.ncbi.nlm.nih.gov/pubmed/33743237?tool=bestpractice.com
Plasma exchange (plasmapheresis)
Plasma exchange should be performed as early as possible:[158]Hughes RA, Wijdicks EF, Barohn R, et al. Practice parameter: immunotherapy for Guillain-Barré syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003 Sep 23;61(6):736-40.
http://n.neurology.org/content/61/6/736.full
http://www.ncbi.nlm.nih.gov/pubmed/14504313?tool=bestpractice.com
Plasma exchange is most effective if started within 7 days of symptom onset, but improvement in outcome has been observed when plasma exchange was initiated up to 30 days after onset.[50]Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017 Feb 27;(2):CD001798.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001798.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28241090?tool=bestpractice.com
[166]McKhann GM, Griffin JW, Cornblath DR, et al. Plasmapheresis and Guillain-Barré syndrome: analysis of prognostic factors and the effect of plasmapheresis. Ann Neurol. 1988 Apr;23(4):347-53.
http://www.ncbi.nlm.nih.gov/pubmed/3382169?tool=bestpractice.com
Plasma exchange should be initiated in parallel with supportive care. Two to five plasma exchanges are often needed, depending on the severity of GBS.[50]Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017 Feb 27;(2):CD001798.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001798.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28241090?tool=bestpractice.com
Patients undergoing plasma exchange should be closely monitored for electrolyte abnormalities and coagulopathies.
The risk of relapse during the first 6 to 12 months after symptom onset is higher with plasma exchange compared with those not treated.[50]Chevret S, Hughes RA, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2017 Feb 27;(2):CD001798.
http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD001798.pub3/full
http://www.ncbi.nlm.nih.gov/pubmed/28241090?tool=bestpractice.com
Rehabilitation
This is recommended in the acute phase. It comprises gentle strengthening involving isometric, isotonic, isokinetic, and manual resistive and progressive resistive exercises. Rehabilitation should be focused on proper limb positioning, posture, orthotics, and nutrition.[151]Hughes RA, Wijdicks EF, Benson E, et al; Multidisciplinary Consensus Group. Supportive care for patients with Guillain-Barré syndrome. Arch Neurol. 2005 Aug;62(8):1194-8.
https://jamanetwork.com/journals/jamaneurology/fullarticle/789059
http://www.ncbi.nlm.nih.gov/pubmed/16087757?tool=bestpractice.com
[11]Leonhard SE, Mandarakas MR, Gondim FAA, et al. Diagnosis and management of Guillain-Barré syndrome in ten steps. Nat Rev Neurol. 2019 Nov;15(11):671-83.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6821638
http://www.ncbi.nlm.nih.gov/pubmed/31541214?tool=bestpractice.com
A multi-disciplinary approach has been shown to improve disability and quality of life, as well as reduce fatigue.[167]Khan F, Amatya B. Rehabilitation interventions in patients with acute demyelinating inflammatory polyneuropathy: a systematic review. Eur J Phys Rehabil Med. 2012 Sep;48(3):507-22.
https://www.minervamedica.it/en/journals/europa-medicophysica/article.php?cod=R33Y2012N03A0507
http://www.ncbi.nlm.nih.gov/pubmed/22820829?tool=bestpractice.com
 ]
[Evidence B]
]
[Evidence B]