Aetiology
Malaria is caused by protozoa from the genus Plasmodium and is transmitted to humans through a bite from one of 40 species of female Anopheles mosquitoes.
Infection may also occur through exposure to infected blood or blood products.[2][3][4]
Five Plasmodium species commonly cause human disease: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. The majority of infections are caused by P falciparum and P vivax, and P falciparum is responsible for the most severe disease.[1]
The distribution of these species is dependent on ecological and behavioural parameters affecting the ability of mosquitoes to transmit them.[21] There are few known animal reservoirs; examples include the chimpanzee for P malariae and the crab-eating (or long-tailed) macaque (Macaca fascicularis) for P knowlesi.
P falciparum is widespread in the tropic regions of Sub-Saharan Africa, certain areas of Southeast Asia, Oceania, and the Amazon basin of South America.[1]
P vivax is predominantly found in parts of Asia, the Americas, parts of Eastern Europe, and North Africa (Horn of Africa).[22] However, more than 80% of cases occur in three countries (Ethiopia, India, and Pakistan). P vivax has a wider geographical distribution than P falciparum, as it can develop in the mosquito at lower temperatures and can survive higher altitudes and cooler climates. Like P ovale, it has a dormant liver stage and can reactivate, causing a relapse of symptoms.
P ovale is found primarily in tropical western and central Africa and islands in the West Pacific.[23]P ovale is now regarded as two sympatric species: P ovale wallikeriand P ovale curtisi.
P malariae has a distribution similar to P falciparum but a lower prevalence.[24][25][26]
P knowlesi is found in certain forested and peri-urban areas of Southeast Asia and is the most common cause of human malaria in Malaysia.[27]
An epidemiological investigation using molecular typing techniques found that Plasmodium simium, a species that is closely related to (but distinct from) P vivax, is responsible for autochthonous malaria in people who live near the Atlantic forest regions in Rio de Janeiro. This parasite was previously thought to be a monkey-specific species. These infections were previously diagnosed as P vivax infection due to their similar clinical presentation.[28] Occasional human infection withP cynomolgi, another monkey-specific species, has also been described.
Risk factors for infection include travel to an endemic area (especially visiting friends and relatives), lack of appropriate chemoprophylaxis, and failure to use an insecticide-treated bed net in an endemic area. Risk factors for severe infection include low host immunity (i.e., individuals living in non-endemic or low-transmission areas), pregnancy, extremes of age, immunocompromise (e.g., underlying HIV infection), and malnutrition.[Figure caption and citation for the preceding image starts]: Female (top) and male (bottom) Anopheles gambiae complex mosquitoes. The female is in the process of egg-laying on a sheet of egg paper. A gambiae is the principal vector of malaria in AfricaCenters for Disease Control and Prevention Image Library/Mary F Adams, MA, MS; used with permission [Citation ends].
Pathophysiology
During a blood meal, an infected female Anopheles mosquito injects 8-15 malarial sporozoites, which rapidly enter hepatocytes. Reproduction by asexual fission (tissue schizogony) takes place to form a pre-erythrocytic schizont. This part of the life-cycle produces no symptoms. After a period of time, 30-40 thousand merozoites are released into the bloodstream to penetrate erythrocytes after attaching via receptors. The time period before merozoites enter the blood is designated the pre-patent period; this is between 7 and 30 days for Plasmodium falciparum, but may be much longer for Plasmodium vivax or Plasmodium ovale because of the possible development of an inactive hypnozoite stage in the liver.[29]
Most merozoites undergo blood schizogony to form trophozoites, evolving to schizonts, which rupture to release new merozoites. These then invade new erythrocytes and the 48-hour (72-hour for Plasmodium malariae and 24 hour for Plasmodium knowlesi) cycle continues, sometimes resulting in periodicity of fever. The rupture of erythrocytes releases toxins that induce the release of cytokines from macrophages, resulting in the symptoms of malaria.[30] Some merozoites mature into larger forms called gametocytes, which reproduce sexually if they are ingested by a mosquito.
The outcome of infection depends on the infecting species, the patient's age and nutritional status, the level of host immunity, and the treatment received.[16][30][31]
Severe disease is more commonly seen with P falciparum, with sequestration (the binding of mature trophozoites to the endothelium of small blood vessels), rosetting (the formation of clumps of infected and uninfected erythrocytes), impaired red cell deformability (in infected and uninfected erythrocytes), cytokine responses, and high levels of parasitaemia (relating to the multiple entry pathways for P falciparum into erythrocytes), which are all likely to contribute to this high mortality.[Figure caption and citation for the preceding image starts]: Illustration of life-cycle of parasites of the genus Plasmodium, which are causal agents of malariaCenters for Disease Control and Prevention Image Library/Alexander J da Silva, PhD/Melanie Moser; used with permission [Citation ends].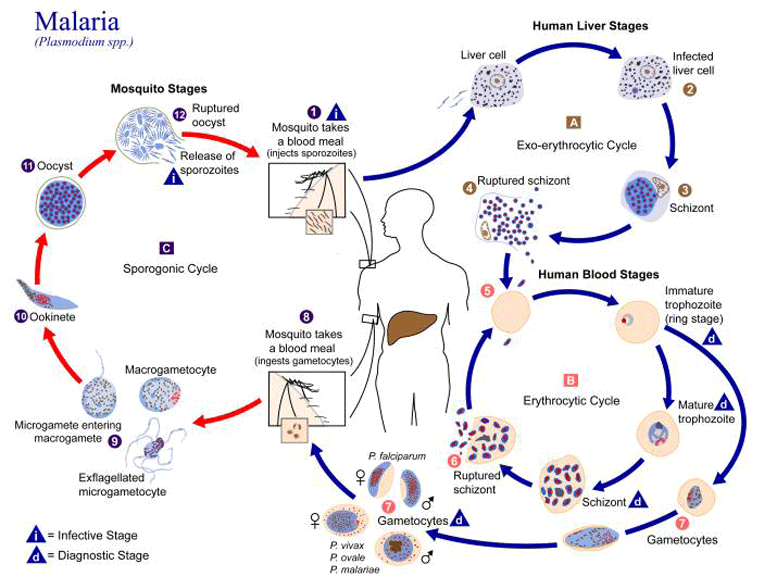
Classification
Taxonomy
There are 5 species of Plasmodium that commonly infect humans:[1]
Plasmodium falciparum, which may cause severe disease or death
Non-falciparum species: Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi, which all usually cause milder illness.
Use of this content is subject to our disclaimer