The approach to treatment depends on whether the constipation is diagnosed as a primary or a secondary condition (e.g., drug-induced constipation, pregnancy, Parkinson's disease). Secondary causes are treated appropriately. In drug-induced constipation, the initial step is to withdraw the drug if possible. Constipation in pregnant women is managed with fibre and laxatives, with consideration of withdrawal of iron supplements.[21]Rao SSC, Qureshi WA, Yan Y, et al. Constipation, hemorrhoids, and anorectal disorders in pregnancy. Am J Gastroenterol. 2022 Oct;117(10s):16-25.
https://journals.lww.com/ajg/fulltext/2022/10001/constipation,_hemorrhoids,_and_anorectal_disorders.4.aspx
[22]Kothari S, Afshar Y, Friedman LS, et al. AGA clinical practice update on pregnancy-related gastrointestinal and liver disease: expert review. Gastroenterology. 2024 Oct;167(5):1033-45.
https://www.doi.org/10.1053/j.gastro.2024.06.014
http://www.ncbi.nlm.nih.gov/pubmed/39140906?tool=bestpractice.com
Initial management of primary constipation will depend on whether the presentation is acute (<3 months) or chronic (≥3 months). Initial management of chronic constipation, irrespective of the cause, focuses on diet and lifestyle changes, ensuring adequate fibre intake and dietary modification. If the condition persists, laxatives are used and potential underlying causes are considered.[Figure caption and citation for the preceding image starts]: Treatment algorithm for constipation (non-opioid induced). PEG: polyethylene glycol (macrogols)Created by BMJ Knowledge Centre from material supplied by Satish Rao, MD and Ashok Attaluri, MD [Citation ends].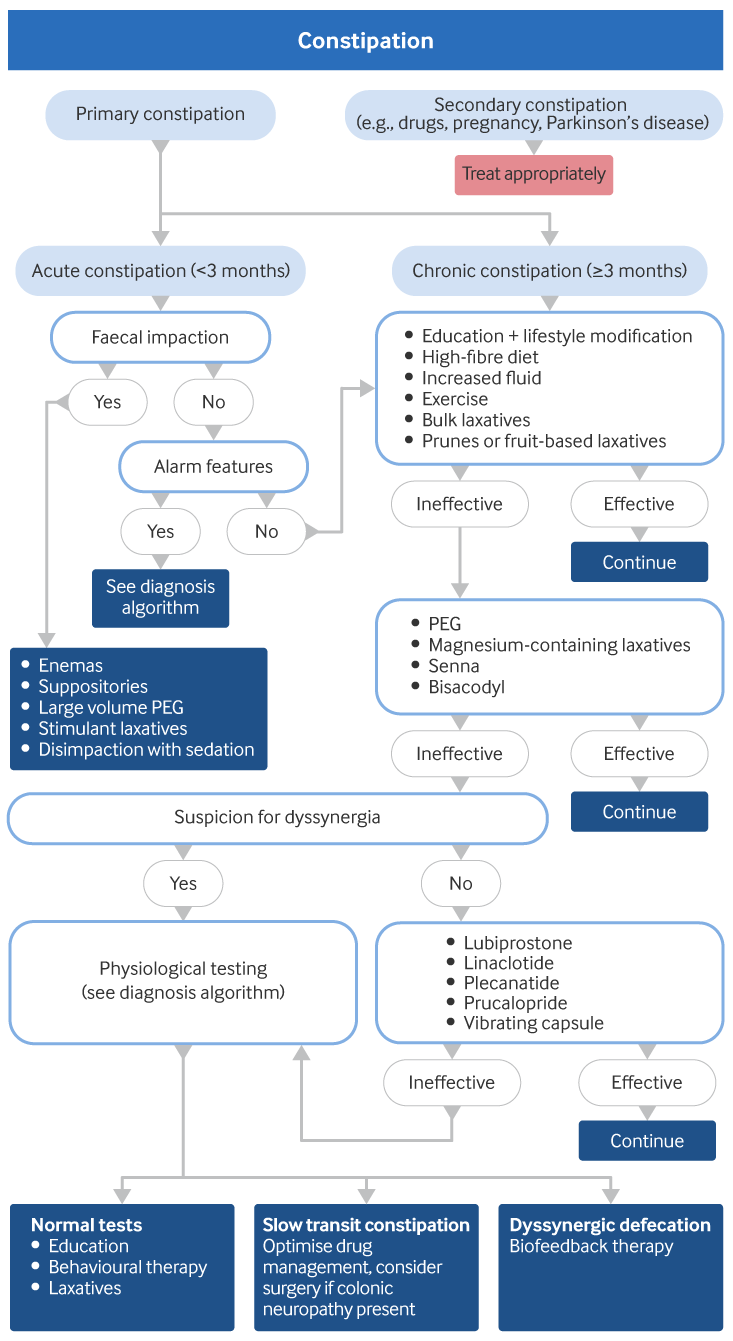
Initial management of acute primary constipation (symptoms <3 months)
When constipation presents acutely, it is important to consider possible secondary causes, including colorectal cancer. Further investigations may be performed to exclude secondary causes. Enemas, suppositories, large volume polyethylene glycol (PEG) solution (also known as macrogols), stimulant laxatives, or disimpaction with sedation may be required if there is faecal impaction. The type of enemas or drugs, and the need for sedation/anaesthesia, are all variables and depend on the clinical setting and individual patient characteristics (e.g., patient age, anxiety, first or recurrent episode). Enemas are contraindicated in patients with neutropenia, thrombocytopenia, intestinal obstruction, recent colorectal or gynecological surgery, inflammatory colitis, toxic megacolon, abdominal infection or inflammation, recent anal or rectal trauma, undiagnosed abdominal pain, or recent pelvic radiotherapy.[51]De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 Jul;38(7):3589-621.
https://link.springer.com/article/10.1007/s12325-021-01766-y
http://www.ncbi.nlm.nih.gov/pubmed/34086265?tool=bestpractice.com
[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
If faecal impaction is absent and secondary causes are excluded, the treatment is the same as for patients with chronic constipation. This includes diet and lifestyle advice, plus laxatives or prunes and/or stool softeners (see section on chronic primary constipation below for further details).
Initial management of chronic primary constipation (symptoms ≥3 months)
Initial steps in the management of chronic primary constipation are:
Patient education
Lifestyle modifications
Correct body posture[53]Wald A. Update on the management of constipation. JAMA. 2019 Dec;322(22):2239-40.
http://www.ncbi.nlm.nih.gov/pubmed/31682683?tool=bestpractice.com
High-fibre diet
Increased fluid
Regular exercise
Bulk laxatives, prunes, or fruit-based laxatives (see section on laxatives below for further details).
Dietary and lifestyle changes may be helpful, and evidence suggests they may be most effective when combined.[18]Serra J, Pohl D, Azpiroz F, et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil. 2020 Feb;32(2):e13762.
https://onlinelibrary.wiley.com/doi/10.1111/nmo.13762
http://www.ncbi.nlm.nih.gov/pubmed/31756783?tool=bestpractice.com
Studies have found conflicting results suggesting that exercise or increased fluid intake alone may not be effective in patients with constipation, but one uncontrolled study combining education on dietary patterns, fluid intake, physical activity, and use of laxatives reported significant improvement in constipation and quality of life.[18]Serra J, Pohl D, Azpiroz F, et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil. 2020 Feb;32(2):e13762.
https://onlinelibrary.wiley.com/doi/10.1111/nmo.13762
http://www.ncbi.nlm.nih.gov/pubmed/31756783?tool=bestpractice.com
Patients are advised to increase their daily dietary fibre. A diet deficient in fibre may lead to constipation, while a high-fibre diet increases stool weight and accelerates colonic transit time.[32]van der Schoot A, Drysdale C, Whelan K, et al. The effect of fiber supplementation on chronic constipation in adults: an updated systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022 Oct;116(4):953-69.
https://www.sciencedirect.com/science/article/pii/S0002916523036146?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35816465?tool=bestpractice.com
[33]Tucker DM, Sandstead HH, Logan GM Jr, et al. Dietary fiber and personality factors as determinants of stool output. Gastroenterology. 1981 Nov;81(5):879-83.
http://www.ncbi.nlm.nih.gov/pubmed/6269944?tool=bestpractice.com
The American Gastroenterological Association-American College of Gastroenterology (AGA-ACG) suggests the use of fibre supplements for the management of chronic idiopathic constipation.[54]Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Am J Gastroenterol. 2023 Jun 1;118(6):936-54.
https://journals.lww.com/ajg/fulltext/2023/06000/american_gastroenterological_association_american.13.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37204227?tool=bestpractice.com
In people with a low calorie intake, an increased daily calorie intake is also advised. It has been shown that inadequate calorie intake can cause constipation.[35]Chun AB, Sokol MS, Kaye WH, et al. Colonic and anorectal function in constipated patients with anorexia nervosa. Am J Gastroenterol. 1997 Oct;92(10):1879-83.
http://www.ncbi.nlm.nih.gov/pubmed/9382057?tool=bestpractice.com
Patients should also be advised on adequate fluid intake, encouraged to get regular non-strenuous exercise, and advised to dedicate time for bowel movements and to avoid postponing bowel movements when an urge for defecation is felt.[34]Muller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol. 2005 Jan;100(1):232-42.
http://www.ncbi.nlm.nih.gov/pubmed/15654804?tool=bestpractice.com
Laxatives for primary constipation
Laxatives are the mainstay of pharmacological treatment and are considered long-term therapy in patients who do not respond to lifestyle or dietary modification. The patient should try each class of drug for a minimum of 6 weeks, and then return for a review to evaluate the response to therapy. If there is no satisfactory clinical response, or if there are troublesome adverse effects, the patient should be switched to another class of drugs.
Dyssynergia should be considered if laxatives are ineffective after approximately 6-8 weeks.[9]Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7.
http://www.gastrojournal.org/article/S0016-5085(12)01545-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/23261064?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Summary of laxatives for chronic constipationCreated by BMJ Knowledge Centre [Citation ends].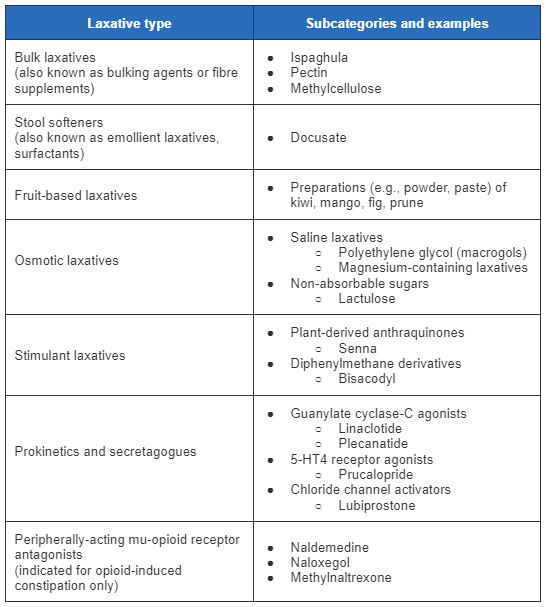
First-line options
Bulk laxatives (fibre supplements), prunes, or fruit-based laxatives are the preferred first-line therapy. In patients who complain of hard stool and straining at stool, stool softeners may be used.
In patients who have occasional loose stool in between episodes of constipation, a bulk laxative may be preferred. In one meta-analysis of 16 randomised controlled trials (RCTs), fibre supplements were shown to be effective at improving chronic constipation (RR 1.48, 95% CI 1.17 to 1.88), with significant effects from ispaghula and pectin supplements at higher doses (>10 g/day) for longer treatment durations (≥4 weeks).[32]van der Schoot A, Drysdale C, Whelan K, et al. The effect of fiber supplementation on chronic constipation in adults: an updated systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022 Oct;116(4):953-69.
https://www.sciencedirect.com/science/article/pii/S0002916523036146?via%3Dihub
http://www.ncbi.nlm.nih.gov/pubmed/35816465?tool=bestpractice.com
Bulk laxatives do not get absorbed systemically and are considered safe for administration during pregnancy.[22]Kothari S, Afshar Y, Friedman LS, et al. AGA clinical practice update on pregnancy-related gastrointestinal and liver disease: expert review. Gastroenterology. 2024 Oct;167(5):1033-45.
https://www.doi.org/10.1053/j.gastro.2024.06.014
http://www.ncbi.nlm.nih.gov/pubmed/39140906?tool=bestpractice.com
Bulk laxatives can produce excessive gas, leading to flatulence and bloating, which may deter patients from continuing treatment.[55]Schiller LR. Review article: the therapy of constipation. Aliment Pharmacol Ther. 2001 Jun;15(6):749-63.
https://onlinelibrary.wiley.com/doi/full/10.1046/j.1365-2036.2001.00982.x?sid=nlm%3Apubmed
http://www.ncbi.nlm.nih.gov/pubmed/11380313?tool=bestpractice.com
Prunes (dried plums) are a natural alternative to laxatives and have been shown in a blinded randomised controlled study to be as effective as ispaghula (ispaghula husk) in improving symptoms of constipation.[56]Attaluri A, Donahoe R, Valestin J, et al. Randomised clinical trial: dried plums (prunes) vs. psyllium for constipation. Aliment Pharmacol Ther. 2011 Apr;33(7):822-8.
http://www.ncbi.nlm.nih.gov/pubmed/21323688?tool=bestpractice.com
Kiwifruit may also be effective and associated with less patient dissatisfaction than prunes or ispaghula, but one systematic review reported evidence is low to very low certainty.[57]Chey SW, Chey WD, Jackson K, et al. Exploratory comparative effectiveness trial of green kiwifruit, psyllium, or prunes in US patients with chronic constipation. Am J Gastroenterol. 2021 Jun;116(6):1304-12.
http://www.ncbi.nlm.nih.gov/pubmed/34074830?tool=bestpractice.com
[58]Eltorki M, Leong R, Ratcliffe EM. Kiwifruit and kiwifruit extracts for treatment of constipation: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2022;2022:7596920.
https://www.hindawi.com/journals/cjgh/2022/7596920
http://www.ncbi.nlm.nih.gov/pubmed/36247043?tool=bestpractice.com
Fruit-based laxatives include preparations (e.g., powder, paste) of kiwi, mango, prunes, or fig.[59]Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol. 2021 Jun;116(6):1156-81.
https://journals.lww.com/ajg/fulltext/2021/06000/efficacy_and_safety_of_over_the_counter_therapies.14.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33767108?tool=bestpractice.com
Studies suggest they are well tolerated with few or no mild gastrointestinal adverse effects reported.
Stool softeners such as docusate (a surfactant thought to hydrate and soften stool by lowering the surface tension at stool’s oil-water interface) are one of the most commonly used non-prescription drugs for treatment of constipation.[59]Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol. 2021 Jun;116(6):1156-81.
https://journals.lww.com/ajg/fulltext/2021/06000/efficacy_and_safety_of_over_the_counter_therapies.14.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33767108?tool=bestpractice.com
However, clinical data have remained inconsistent and there have been no new efficacy studies since 2004. In one study, docusate was found to be no more effective than placebo and significantly inferior to ispaghula.[60]Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005 Apr;100(4):936-71.
http://www.ncbi.nlm.nih.gov/pubmed/15784043?tool=bestpractice.com
Adverse effects of stool softeners include a bitter taste in the mouth, nausea, diarrhoea, and cramping.
Second-line options
If there is no clinical response (defined as patient satisfaction with bowel habits and associated symptoms) after at least 6 weeks of therapy, and no suspicion of dyssynergia, osmotic laxatives such as lactulose, PEG (macrogols), or magnesium-containing laxatives are considered.[54]Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Am J Gastroenterol. 2023 Jun 1;118(6):936-54.
https://journals.lww.com/ajg/fulltext/2023/06000/american_gastroenterological_association_american.13.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37204227?tool=bestpractice.com
[61]Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev. 2010 Jul 7;(7):CD007570.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007570.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/20614462?tool=bestpractice.com
[62]Belsey JD, Geraint M, Dixon TA. Systematic review and meta analysis: polyethylene glycol in adults with non-organic constipation. Int J Clin Pract. 2010 Jun;64(7):944-55.
http://www.ncbi.nlm.nih.gov/pubmed/20584228?tool=bestpractice.com
Often a single class of laxative is used as much as possible. One meta-analysis has shown that PEG (macrogols) is better than lactulose in improving outcome measures of stool frequency per week, form of stool, relief of abdominal pain, and the need for additional products.[61]Lee-Robichaud H, Thomas K, Morgan J, et al. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst Rev. 2010 Jul 7;(7):CD007570.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007570.pub2/full
http://www.ncbi.nlm.nih.gov/pubmed/20614462?tool=bestpractice.com
Based on the evidence, AGA-ACG made a strong recommendation for the use of PEG (either following or in combination with fibre supplements) in the management of chronic idiopathic constipation, and weak recommendations for the use of lactulose or magnesium oxide.[54]Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Am J Gastroenterol. 2023 Jun 1;118(6):936-54.
https://journals.lww.com/ajg/fulltext/2023/06000/american_gastroenterological_association_american.13.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37204227?tool=bestpractice.com
Lactulose and PEG are considered safe for administration during pregnancy.[22]Kothari S, Afshar Y, Friedman LS, et al. AGA clinical practice update on pregnancy-related gastrointestinal and liver disease: expert review. Gastroenterology. 2024 Oct;167(5):1033-45.
https://www.doi.org/10.1053/j.gastro.2024.06.014
http://www.ncbi.nlm.nih.gov/pubmed/39140906?tool=bestpractice.com
The main adverse effects of osmotic laxatives include bloating, diarrhoea, epigastric pain, flatulence, nausea, vomiting, and, rarely, hypernatraemia and hypokalaemia. Common adverse effects of magnesium-containing laxatives include nausea, diarrhoea, and hypermagnesaemia.
Stimulant laxatives, such as bisacodyl and senna, are another option at this stage. They may also be used as rescue laxatives in patients using another class of laxatives (to be used if the patient does not have a bowel movement for 3 days).[63]Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 2011 Jul;9(7):577-83.
http://www.ncbi.nlm.nih.gov/pubmed/21440672?tool=bestpractice.com
Systematic reviews have found that while stimulant laxatives are effective, diarrhoea and abdominal pain are common, which may impact their tolerability.[59]Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol. 2021 Jun;116(6):1156-81.
https://journals.lww.com/ajg/fulltext/2021/06000/efficacy_and_safety_of_over_the_counter_therapies.14.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33767108?tool=bestpractice.com
[64]Luthra P, Camilleri M, Burr NE, et al. Efficacy of drugs in chronic idiopathic constipation: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2019 Nov;4(11):831-44.
http://www.ncbi.nlm.nih.gov/pubmed/31474542?tool=bestpractice.com
AGA-ACG recommend bisacodyl for daily use up to 4 weeks or as a rescue therapy. A gradual increase in dose is suggested to improve tolerability. They also suggest senna for the management of chronic idiopathic constipation.[54]Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Am J Gastroenterol. 2023 Jun 1;118(6):936-54.
https://journals.lww.com/ajg/fulltext/2023/06000/american_gastroenterological_association_american.13.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37204227?tool=bestpractice.com
Stimulant laxatives should be avoided during pregnancy as safety data are conflicting.[21]Rao SSC, Qureshi WA, Yan Y, et al. Constipation, hemorrhoids, and anorectal disorders in pregnancy. Am J Gastroenterol. 2022 Oct;117(10s):16-25.
https://journals.lww.com/ajg/fulltext/2022/10001/constipation,_hemorrhoids,_and_anorectal_disorders.4.aspx
One systematic review evaluating RCTs of non-prescription constipation drugs concluded that PEG and senna were supported by good evidence.[59]Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol. 2021 Jun;116(6):1156-81.
https://journals.lww.com/ajg/fulltext/2021/06000/efficacy_and_safety_of_over_the_counter_therapies.14.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33767108?tool=bestpractice.com
Fibre supplements (ispaghula); fruit-based laxatives (preparations of kiwi, mango, prunes, fig); magnesium salts; stimulants (bisacodyl); and yogurt with galacto-oligosaccharides/prunes/linseed oil were recommended with moderate evidence. There was insufficient evidence for docusate, inulin, fructose-oligosaccharides, and polydextrose.[59]Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol. 2021 Jun;116(6):1156-81.
https://journals.lww.com/ajg/fulltext/2021/06000/efficacy_and_safety_of_over_the_counter_therapies.14.aspx
http://www.ncbi.nlm.nih.gov/pubmed/33767108?tool=bestpractice.com
Third-line options
If symptoms persist after at least 6 weeks of traditional laxatives and there is no suspicion of dyssynergia, newer prescription agents may be considered. Options include lubiprostone, linaclotide, plecanatide, or prucalopride. These drugs are usually used as monotherapy and not in combination with other laxatives. AGA-ACG recommend linaclotide, plecanatide, or prucalopride and suggest lubiprostone for patients with chronic idiopathic constipation that does not respond to non-prescription agents.[54]Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association-American College of Gastroenterology clinical practice guideline: pharmacological management of chronic idiopathic constipation. Am J Gastroenterol. 2023 Jun 1;118(6):936-54.
https://journals.lww.com/ajg/fulltext/2023/06000/american_gastroenterological_association_american.13.aspx
http://www.ncbi.nlm.nih.gov/pubmed/37204227?tool=bestpractice.com
They are not recommended for pregnant patients as there is a lack of safety data for use in pregnancy.
Lubiprostone (a chloride channel activator) has been shown to be more effective than placebo in increasing the number of spontaneous bowel movements, decreasing straining, improving stool consistency, and relieving symptoms of chronic constipation.[65]Johanson JF, Panas R, Holland PC, et al. Long-term efficacy of lubiprostone for the treatment of chronic constipation. Gastroenterology. 2006;130(suppl 2):M1171.[66]Johanson JF, Gargano MA, Holland PC, et al. Initial and sustained effects of lubiprostone, a chloride channel-2 (ClC2) activator for the treatment of constipation: data from a 4-week phase III study. Am J Gastroenterol. 2005;100(suppl 9):S328.[67]Barish CF, Drossman D, Johanson JF, et al. Efficacy and safety of lubiprostone in patients with chronic constipation. Dig Dis Sci. 2010 Apr;55(4):1090-7.
http://www.ncbi.nlm.nih.gov/pubmed/20012484?tool=bestpractice.com
[68]Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut. 2011 Feb;60(2):209-18.
http://www.ncbi.nlm.nih.gov/pubmed/21205879?tool=bestpractice.com
The most common adverse events include nausea (31%), headache (13%), diarrhoea (13%), abdominal pain (7%), and distention (7%). Lubiprostone has been withdrawn from the UK market for commercial reasons.
Linaclotide and plecanatide are guanylate cyclase-C agonists. This class of drugs has been shown to be more effective than placebo in clinical trials, where it has demonstrated rapid and sustained improvements of bowel habits, bowel and abdominal symptoms and quality of life in patients with chronic constipation.[69]Johnston JM, Kurtz CB, Macdougall JE, et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology. 2010 Dec;139(6):1877-1886.e2.
http://www.ncbi.nlm.nih.gov/pubmed/20801122?tool=bestpractice.com
[70]Lembo AJ, Schneier HA, Shiff SJ, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med. 2011 Aug 11;365(6):527-36.
http://www.ncbi.nlm.nih.gov/pubmed/21830967?tool=bestpractice.com
[71]DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol. 2017 Nov;10(11):837-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5673020
http://www.ncbi.nlm.nih.gov/pubmed/29147135?tool=bestpractice.com
Linaclotide has been shown to accelerate gut transit in healthy people and in female patients with irritable bowel syndrome-constipation.[72]Currie MG, Kurtz CB, Mahajan-Miklos S, et al. Effects of a single dose administration of MD-1100 on safety, tolerability, exposure, and stool consistency in healthy subjects. Am J Gastroenterol. 2005;100:S328.[73]Johnston JM, Drossman DA, Lembo A, et al. Late-breaking abstract 65A: Linaclotide improves bowel habits and patient reported outcomes in subjects with chronic constipation. Am J Gastroenterol. 2006;101(suppl 2):S470-S494. Plecanatide has similarly demonstrated to increase intracellular cyclic guanosine monophosphate (cGMP) and promote luminal secretion through the CFTR receptor to facilitate bowel movements.[71]DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap Adv Gastroenterol. 2017 Nov;10(11):837-51.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5673020
http://www.ncbi.nlm.nih.gov/pubmed/29147135?tool=bestpractice.com
Both linaclotide and plecanatide appear to have few adverse effects.
Prucalopride is a 5-HT4 receptor agonist with prokinetic properties. Prucalopride was the most effective laxative at 12 weeks according to one meta-analysis.[64]Luthra P, Camilleri M, Burr NE, et al. Efficacy of drugs in chronic idiopathic constipation: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2019 Nov;4(11):831-44.
http://www.ncbi.nlm.nih.gov/pubmed/31474542?tool=bestpractice.com
Headache, nausea, abdominal pain, and diarrhoea were reported by over 10% of the 4000 patients in the three phase 3 trials.[74]Camilleri M, Kerstens R, Rykx A, et al. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008 May 29;358(22):2344-54.
http://www.nejm.org/doi/full/10.1056/NEJMoa0800670#t=article
http://www.ncbi.nlm.nih.gov/pubmed/18509121?tool=bestpractice.com
[75]Quigley EM, Vandeplassche L, Kerstens R, et al. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation - a 12-week, randomized, double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2009 Feb 1;29(3):315-28.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2036.2008.03884.x/full
http://www.ncbi.nlm.nih.gov/pubmed/19035970?tool=bestpractice.com
[76]Yiannakou Y, Piessevaux H, Bouchoucha M, et al. A randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the efficacy, safety, and tolerability of prucalopride in men with chronic constipation. Am J Gastroenterol. 2015 May;110(5):741-8.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4424376
http://www.ncbi.nlm.nih.gov/pubmed/25869393?tool=bestpractice.com
No clinically relevant cardiac adverse effects were reported. In one meta-analysis of 14 RCTs (total of 4328 patients), a lower daily dose obtained the maximum number of spontaneous weekly bowel movements, compared with higher doses, and higher doses were associated with more treatment-emergent adverse events.[77]Yang T, Wang K, Cao Y, et al. Different doses of prucalopride in treating chronic idiopathic constipation: a meta-analysis and Bayesian analysis. BMJ Open. 2021 Feb;11(2):e039461.
https://bmjopen.bmj.com/content/11/2/e039461.long
http://www.ncbi.nlm.nih.gov/pubmed/33589446?tool=bestpractice.com
In the US, the FDA has approved a vibrating capsule for treatment of chronic constipation in adults who have not experienced symptom improvement from laxatives after at least 1 month. The vibrating capsule is a non-pharmacological device that mechanically induces colonic contractions.[78]Ron Y, Halpern Z, Safadi R, et al. Safety and efficacy of the vibrating capsule, an innovative non-pharmacological treatment modality for chronic constipation. Neurogastroenterol Motil. 2015 Jan;27(1):99-104.
http://www.ncbi.nlm.nih.gov/pubmed/25484196?tool=bestpractice.com
In a double-blind phase 3 trial of patients with chronic constipation, a significantly higher proportion of patients who received the vibrating capsule experienced ≥1 complete spontaneous bowel movements per week, compared with those who received the sham placebo capsule (39.3% versus 22.1%).[79]Rao SSC, Quigley EMM, Chey WD, et al. Randomized placebo-controlled phase 3 trial of vibrating capsule for chronic constipation. Gastroenterology. 2023 Jun;164(7):1202-10.e6.
https://www.gastrojournal.org/article/S0016-5085(23)00149-X/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36822371?tool=bestpractice.com
Studies also reported improvements in quality of life, and mild gastrointestinal adverse effects.[79]Rao SSC, Quigley EMM, Chey WD, et al. Randomized placebo-controlled phase 3 trial of vibrating capsule for chronic constipation. Gastroenterology. 2023 Jun;164(7):1202-10.e6.
https://www.gastrojournal.org/article/S0016-5085(23)00149-X/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/36822371?tool=bestpractice.com
[80]Zhu JH, Qian YY, Pan J, et al. Efficacy and safety of vibrating capsule for functional constipation (VICONS): a randomised, double-blind, placebo-controlled, multicenter trial. EClinicalMedicine. 2022 May;47:101407.
https://www.thelancet.com/action/showPdf?pii=S2589-5370%2822%2900137-7
http://www.ncbi.nlm.nih.gov/pubmed/35518121?tool=bestpractice.com
If there has been no bowel movement within ≥3 consecutive days of using the vibrating capsule, consider adjunctive laxative therapy.
Enemas, suppositories, and digital maneuvers are not normally recommended for the management of chronic constipation. However despite lack of robust evidence, they are commonly used. Suppositories are not associated with any obvious risk, but enemas should be avoided in patients with fluid or electrolyte imbalance.[18]Serra J, Pohl D, Azpiroz F, et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil. 2020 Feb;32(2):e13762.
https://onlinelibrary.wiley.com/doi/10.1111/nmo.13762
http://www.ncbi.nlm.nih.gov/pubmed/31756783?tool=bestpractice.com
Opioid-induced constipation
Opioid use should be reviewed as appropriate. Other underlying causes or factors that may contribute to constipation should also be considered and excluded.[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
Traditional laxatives are recommended as first-line therapy for opioid-induced constipation. Osmotic or stimulant laxatives are preferred.[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
If response to a single agent is inadequate, some guidelines recommend combining an osmotic and stimulant laxative before escalating therapy.[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
Docusate is not typically recommended due to lack of evidence.[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
Fibre (including bulk laxatives like ispaghula) may be helpful in patients with a fibre-deficient diet, but it is typically not recommended for treatment of opioid-induced constipation because it does not affect colonic motility.[51]De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 Jul;38(7):3589-621.
https://link.springer.com/article/10.1007/s12325-021-01766-y
http://www.ncbi.nlm.nih.gov/pubmed/34086265?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
Peripherally acting mu-opioid receptor antagonists such as naldemedine, naloxegol, and methylnaltrexone are recommended for laxative-refractory opioid-induced constipation.[18]Serra J, Pohl D, Azpiroz F, et al. European society of neurogastroenterology and motility guidelines on functional constipation in adults. Neurogastroenterol Motil. 2020 Feb;32(2):e13762.
https://onlinelibrary.wiley.com/doi/10.1111/nmo.13762
http://www.ncbi.nlm.nih.gov/pubmed/31756783?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
They can be used as monotherapy, but are often used in combination with an osmotic or stimulant laxative.[82]National Institute for Health and Care Excellence. Naldemedine for treating opioid-induced constipation. Sep 2020 [internet publication].
https://www.nice.org.uk/guidance/ta651
Data to support naldemedine come from one phase 2 and three phase 3 trials.[83]Webster LR, Yamada T, Arjona Ferreira JC. A phase 2b, Randomized, double-blind placebo-controlled study to evaluate the efficacy and safety of naldemedine for the treatment of opioid-induced constipation in patients with chronic noncancer pain. Pain Med. 2017 Dec;18(12):2350-60.
https://academic.oup.com/painmedicine/article/18/12/2350/3078985?login=false
http://www.ncbi.nlm.nih.gov/pubmed/28371937?tool=bestpractice.com
[84]Hale M, Wild J, Reddy J, et al. Naldemedine versus placebo for opioid-induced constipation (COMPOSE-1 and COMPOSE-2): two multicentre, phase 3, double-blind, randomised, parallel-group trials. Lancet Gastroenterol Hepatol. 2017 Aug;2(8):555-64.
http://www.ncbi.nlm.nih.gov/pubmed/28576452?tool=bestpractice.com
[85]Webster LR, Nalamachu S, Morlion B, et al. Long-term use of naldemedine in the treatment of opioid-induced constipation in patients with chronic noncancer pain: a randomized, double-blind, placebo-controlled phase 3 study. Pain. 2018 May;159(5):987-94.
https://journals.lww.com/pain/fulltext/2018/05000/long_term_use_of_naldemedine_in_the_treatment_of.18.aspx
http://www.ncbi.nlm.nih.gov/pubmed/29419653?tool=bestpractice.com
The efficacy of naloxegol was shown in one phase 2 trial and two phase 3 RCTs.[86]Webster L, Dhar S, Eldon M, et al. A phase 2, double-blind, randomized, placebo-controlled, dose-escalation study to evaluate the efficacy, safety, and tolerability of naloxegol in patients with opioid-induced constipation. Pain. 2013 Sep;154(9):1542-50.
http://www.ncbi.nlm.nih.gov/pubmed/23726675?tool=bestpractice.com
[87]Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96.
http://www.ncbi.nlm.nih.gov/pubmed/24896818?tool=bestpractice.com
[88]Chey WD, Brenner DM, Cash BD, et al. Efficacy and safety of naloxegol in patients with chronic non-cancer pain who experience opioid induced constipation: a pooled analysis of two global, randomized controlled studies. J Pain Res. 2023;16:2943-53.
https://www.dovepress.com/efficacy-and-safety-of-naloxegol-in-patients-with-chronic-non-cancer-p-peer-reviewed-fulltext-article-JPR
http://www.ncbi.nlm.nih.gov/pubmed/37664485?tool=bestpractice.com
The quality of evidence supporting methylnaltrexone is not as robust.[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
Out of five RCTs, only three examined an outcome of ≥3 spontaneous bowel movements per week, and most studies were in patients with cancer. However, the subcutaneous formulation of methylnaltrexone is an advantage for patients who cannot tolerate oral drugs.[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
The most common adverse effects of methylnaltrexone are abdominal cramping and flatulence. Concerns have been raised about severe abdominal pain and bowel perforation in patients with advanced cancer who were receiving methylnaltrexone for opioid-induced constipation. These concerns led the FDA to issue a warning for physicians in the US to use caution in administering methylnaltrexone to patients with known or suspected lesions in the intestinal wall, and to discontinue treatment with methylnaltrexone immediately if gastrointestinal symptoms worsen.[89]US Food and Drug Administration (FDA). MedWatch safety information: Relistor (methylnaltrexone bromide) subcutaneous injections. August 2013 [internet publication].
https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021964s011lbl.pdf
Regardless, a pooled analysis showed reduced all-cause mortality in cancer and non-cancer patients with opioid-induced constipation who took methylnaltrexone compared to placebo.[90]Webster LR, Brenner D, Israel RJ, et al. Reductions in all-cause mortality associated with the use of methylnaltrexone for opioid-induced bowel disorders: a pooled analysis. Pain Med. 2023 Mar;24(3):341-50.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9977130
http://www.ncbi.nlm.nih.gov/pubmed/36102822?tool=bestpractice.com
One Cochrane review on peripherally acting mu-opioid receptor antagonists for opioid-induced bowel dysfunction concluded that moderate-certainty evidence supports the efficacy of naldemedine for people with cancer and subcutaneous methylnaltrexone for people receiving palliative care.[91]Candy B, Jones L, Vickerstaff V, et al. Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care. Cochrane Database Syst Rev. 2022 Sep;9(9):CD006332.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006332.pub4/full
http://www.ncbi.nlm.nih.gov/pubmed/36106667?tool=bestpractice.com
Lubiprostone is also FDA approved for treatment of opioid-induced constipation in adults with non-cancer pain, and phase 3 trials found lubiprostone was more effective than placebo for opioid-induced constipation.[92]Webster LR, Brewer RP, Lichtlen P, et al. Efficacy of lubiprostone for the treatment of opioid-induced constipation, analyzed by opioid class. Pain Med. 2018 Jun;19(6):1195-205.
https://academic.oup.com/painmedicine/article/19/6/1195/4396351?login=false
http://www.ncbi.nlm.nih.gov/pubmed/29897589?tool=bestpractice.com
However, in practice, peripherally acting mu-opioid receptor antagonists and laxatives (e.g., PEG or magnesium compounds) are preferred due to the increased cost of lubiprostone. Other prokinetics and secretagogues (e.g., linaclotide, plecanatide, prucalopride) are not FDA approved for opioid-induced constipation.
Patients with opioid-induced constipation should also be advised on appropriate dietary and lifestyle modifications, adjusted according to the clinical setting and underlying conditions.[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
There is a lack of evidence to support the use of enemas and suppositories for treatment of opioid-induced constipation, and their use is limited by inconvenience and safety concerns.[51]De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 Jul;38(7):3589-621.
https://link.springer.com/article/10.1007/s12325-021-01766-y
http://www.ncbi.nlm.nih.gov/pubmed/34086265?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
However, evacuation measures including enemas, suppositories, large volume PEG solution, stimulant laxatives, or disimpaction with sedation may be required if there is faecal impaction.[51]De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 Jul;38(7):3589-621.
https://link.springer.com/article/10.1007/s12325-021-01766-y
http://www.ncbi.nlm.nih.gov/pubmed/34086265?tool=bestpractice.com
[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
[81]Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute guideline on the medical management of opioid-induced constipation. Gastroenterology. 2019 Jan;156(1):218-26.
https://www.gastrojournal.org/article/S0016-5085(18)34782-6/fulltext?referrer=https%3A%2F%2Fpubmed.ncbi.nlm.nih.gov%2F
http://www.ncbi.nlm.nih.gov/pubmed/30340754?tool=bestpractice.com
Enemas are contraindicated in patients with neutropenia, thrombocytopenia, intestinal obstruction, recent colorectal or gynecological surgery, inflammatory colitis, toxic megacolon, abdominal infection or inflammation, recent anal or rectal trauma, undiagnosed abdominal pain, or recent pelvic radiotherapy.[51]De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 Jul;38(7):3589-621.
https://link.springer.com/article/10.1007/s12325-021-01766-y
http://www.ncbi.nlm.nih.gov/pubmed/34086265?tool=bestpractice.com
[52]Larkin PJ, Cherny NI, La Carpia D, et al. Diagnosis, assessment and management of constipation in advanced cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2018 Oct 1;29(suppl 4):iv111-25.
https://www.annalsofoncology.org/article/S0923-7534(19)31697-7/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/30016389?tool=bestpractice.com
Suspected dyssynergia
Patients with suspected dyssynergia would have already been treated in the same way as patients with chronic primary constipation, with education, lifestyle modifications, high-fibre diet, bulk laxatives, increased fluid, and exercise. They may have also been treated with osmotic or stimulant laxatives and/or stool softeners.
When these are ineffective, the possibility of dyssynergia should be considered.[9]Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7.
http://www.gastrojournal.org/article/S0016-5085(12)01545-4/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/23261064?tool=bestpractice.com
Associated symptoms include the sensation of anal blockage, straining, and the use of digital manoeuvres to assist defecation. The diagnosis is confirmed with physiological testing. See Diagnosis approach.
Biofeedback (bowel retraining) has been found to be effective in treating dyssynergia.[93]Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007 Mar;5(3):331-8.
http://www.ncbi.nlm.nih.gov/pubmed/17368232?tool=bestpractice.com
[94]Rao SS, Kinkade K, Miller MJ, et al. Randomized controlled trial of long term outcome of biofeedback therapy (BT) for dyssynergic defecation. Am J Gastroenterol. 2005;100:386.[95]Heymen S, Wexner SD, Vickers D, et al. Prospective, randomized trial comparing for biofeedback techniques for patients with constipation. Dis Colon Rectum. 1999 Nov;42(11):1388-93.
http://www.ncbi.nlm.nih.gov/pubmed/10566525?tool=bestpractice.com
[96]Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006 Mar;130(3):657-64.
http://www.ncbi.nlm.nih.gov/pubmed/16530506?tool=bestpractice.com
Biofeedback techniques used to improve coordination of abdominal and anorectal muscles include:
Diaphragmatic muscle training with simulated defecation
Manometric guided pelvic floor retraining
Simulated defecation training.
Three RCTs have compared biofeedback therapy with either sham feedback or pharmacological therapy or placebo; all three trials found biofeedback therapy was more effective.[93]Rao SS, Seaton K, Miller M, et al. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin Gastroenterol Hepatol. 2007 Mar;5(3):331-8.
http://www.ncbi.nlm.nih.gov/pubmed/17368232?tool=bestpractice.com
[95]Heymen S, Wexner SD, Vickers D, et al. Prospective, randomized trial comparing for biofeedback techniques for patients with constipation. Dis Colon Rectum. 1999 Nov;42(11):1388-93.
http://www.ncbi.nlm.nih.gov/pubmed/10566525?tool=bestpractice.com
[96]Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006 Mar;130(3):657-64.
http://www.ncbi.nlm.nih.gov/pubmed/16530506?tool=bestpractice.com
Two RCTs reported sustained (1 year) improvement of symptoms and colorectal function, confirming the long-term efficacy of biofeedback therapy.[94]Rao SS, Kinkade K, Miller MJ, et al. Randomized controlled trial of long term outcome of biofeedback therapy (BT) for dyssynergic defecation. Am J Gastroenterol. 2005;100:386.[96]Chiarioni G, Whitehead WE, Pezza V, et al. Biofeedback is superior to laxatives for normal transit constipation due to pelvic floor dyssynergia. Gastroenterology. 2006 Mar;130(3):657-64.
http://www.ncbi.nlm.nih.gov/pubmed/16530506?tool=bestpractice.com
One study compared home biofeedback therapy to standard 6-session office-based biofeedback therapy over 3 months and reported that approximately 70% of patients in both groups met primary outcomes (i.e., normalisation of dyssynergia and improvement in the number of complete spontaneous bowel movements per week).[97]Rao SSC, Valestin JA, Xiang X, et al. Home-based versus office-based biofeedback therapy for constipation with dyssynergic defecation: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2018 Nov;3(11):768-77.
http://www.ncbi.nlm.nih.gov/pubmed/30236904?tool=bestpractice.com
Sixty per cent of patients with dyssynergic defecation have impaired rectal sensation, and rectal sensory conditioning provides additional therapeutic benefit.[94]Rao SS, Kinkade K, Miller MJ, et al. Randomized controlled trial of long term outcome of biofeedback therapy (BT) for dyssynergic defecation. Am J Gastroenterol. 2005;100:386.[98]Rao SS, Enck P, Loening-Baucke V. Biofeedback therapy for defecation disorders. Dig Dis. 1997;15(suppl 1):78-92.
http://www.ncbi.nlm.nih.gov/pubmed/9177947?tool=bestpractice.com
One RCT found that barostat-assisted sensory training was superior to syringe-assisted sensory training.[99]Rao SSC, Yan Y, Erdogan A, et al. Barostat or syringe-assisted sensory biofeedback training for constipation with rectal hyposensitivity: a randomized controlled trial. Neurogastroenterol Motil. 2022 Mar;34(3):e14226.
https://onlinelibrary.wiley.com/doi/10.1111/nmo.14226
http://www.ncbi.nlm.nih.gov/pubmed/34431186?tool=bestpractice.com
Patients with confirmed dyssynergia are gradually weaned off laxatives. Lifestyle and dietary changes are continued, including adequate daily fluids and increased dietary fibre.
Persistent chronic constipation despite medical therapy and lifestyle measures
Patients with persistent symptoms (lasting several months to years) that are unresponsive to medical treatment can be referred to a specialised centre for consideration of surgery, which could include colectomy and caecostomy. Such patients undergo evaluation of upper gut and small bowel motility, along with a detailed assessment of colonic motility with colonic manometry. Surgery is reserved for refractory patients with severe colonic neuropathy and relatively preserved gastric and small bowel motility. All other patients continued on medical management.
If patients have failed all therapies and have evidence for motility dysfunction not limited to the colon (in other words, if they have gastric or small bowel dysmotility), surgery is unlikely to be of benefit. Further management involves a multi-disciplinary approach, including addressing nutrition, behavioural therapy, biofeedback, psychotherapy, and cyclical laxatives. The evidence for this type of therapy is mostly anecdotal.
Caecostomy is generally preferred in institutionalised patients, and in patients with neurological lesions, in whom there seems to be a high success rate, with satisfactory results ranging from 40% to 78%.[100]Lees NP, Hodson P, Hill J, et al. Long-term results of the antegrade continent enema procedure for constipation in adults. Colorectal Dis. 2004 Sep;6(5):362-8.
http://www.ncbi.nlm.nih.gov/pubmed/15335371?tool=bestpractice.com
Several colectomy techniques have been advocated for the treatment of chronic constipation, including segmental colectomy, ileorectal anastomosis, ileosigmoid anastomosis, caecorectal anastomosis, ileoanal anastomosis with proctocolectomy, and pouch formation or ileostomy.[101]Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991 Oct;214(4):403-11.
http://www.ncbi.nlm.nih.gov/pubmed/1953096?tool=bestpractice.com
Surgery has also been shown to be associated with a higher degree of patient satisfaction.[102]Arebi N, Kalli T, Howson W, et al. Systematic review of abdominal surgery for chronic idiopathic constipation. Colorectal Dis. 2011 Dec;13(12):1335-43.
http://www.ncbi.nlm.nih.gov/pubmed/20969711?tool=bestpractice.com
Most of these surgeries can be carried out laparoscopically.

