Aetiology
Hepatitis B virus (HBV) is an enveloped, non-cytopathic, hepatotropic, and highly infectious DNA virus that belongs in the hepadnaviruses family.[22][23] The outer envelope of the virus contains three related surface antigens, the most abundant of which is the S protein (hepatitis B surface antigen [HBsAg]). The development of cellular and humoral immunity to HBsAg is protective. Inside the envelope is the viral nucleocapsid, or core, which contains partially double-stranded circular DNA (hepatitis B core antigen [HBcAg]). HBcAg-derived peptides induce a crucial host cellular immune response against HBV. Hepatitis B e antigen, a hepatitis B viral protein, serves as a marker for active replication, but its function is unknown. The X protein may play a role in development of hepatocellular carcinoma. DNA polymerase serves a reverse-transcriptase function for the synthesis of both negative and positive strands of HBV DNA.[22][23]
Pathophysiology
The virus does not directly kill hepatocytes.[23] The host's immune response to viral antigens is thought to be the cause of the liver injury in hepatitis B virus (HBV) infection.[24] The cellular immune response, rather than the humoral immune response, seems to be primarily involved in disease pathogenesis. Induction of antigen-specific T-lymphocyte response is thought to occur when host T lymphocytes are presented with viral epitopes by antigen-presenting cells in lymphoid organs. These antigen-specific T cells mature and expand and then migrate to the liver. In acute HBV infection, most HBV DNA is cleared from hepatocytes through non-cytocidal effects of inflammatory byproducts of CD8+ T lymphocytes, stimulated by CD4+ T lymphocytes, notably interferon-gamma and tumour necrosis factor-alfa. These cause down-regulation of viral replication, and trigger direct lysis of infected hepatocytes by HBV-specific CD8+ cytotoxic T cells.[25] In contrast, people with chronic HBV infection display weak, infrequent, and narrowly focused HBV-specific T-cell responses, and the majority of mononuclear cells in livers of chronic HBV-infected people are non-antigen-specific.[26]
[Figure caption and citation for the preceding image starts]: Life cycle of HBVFrom Ganem D, Prince AM. Hepatitis B virus infection - natural history and clinical consequences. N Engl J Med. 2004; 350:1118-1129; used with permission [Citation ends].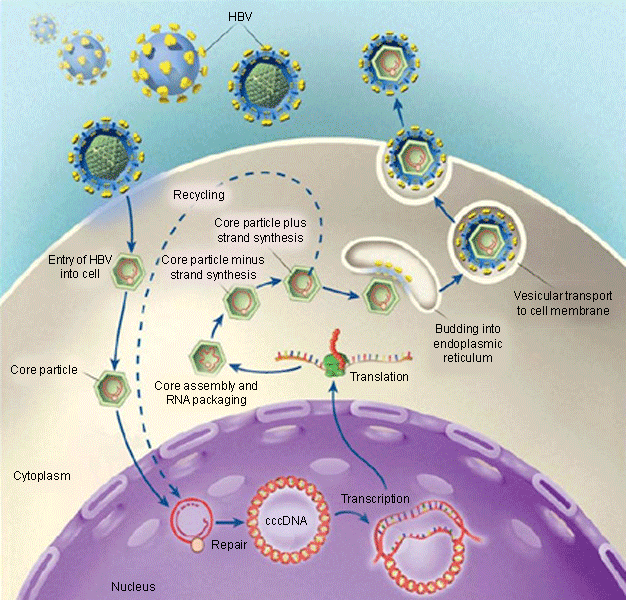
Due to the presence of HBV in extrahepatic sites, as well as the presence of covalently closed circular DNA (cccDNA) within hepatocytes, eradication of the virus is an unrealistic goal based on the currently available drugs. Covalently closed circular DNA serves as a template for transcription of pregenomic messenger RNA, a vital initial step in HBV replication.[27][28][29][30] The continued presence of cccDNA within hepatocytes is considered as a marker of viral persistence. Unfortunately, current therapies have not been effective in eradicating cccDNA and are only able to decrease levels.[31][32][33][34][35][36][37] Persistence of even low levels of cccDNA in the hepatocyte nucleus has been shown to correlate with viral rebound after discontinuation of therapy. In addition, the integration of HBV DNA to the hepatocyte nucleus during replication process could explain increased risk for hepatocellular carcinoma. Furthermore, co-infection with hepatitis C virus (HCV) can synergistically increase the rate of fibrosis, cirrhosis, and hepatocellular cancer, because both HBV and HCV can infect the same hepatocyte independently.[38][39][40][41][42]
Classification
HBV genotype
Hepatitis B virus (HBV) genotypes are based on 8% intertypic variation of complete nucleotide sequence of the genome, and are distributed geographically.[1] Data show that HBV genotypes may play a role in HBV-related liver disease progression and response to interferon therapy.[2] Studies have shown increased hepatitis B e antigen (HBeAg) seroconversion with both genotype A and genotype B, although one study demonstrated that the improvement in HBeAg seroconversion was limited to only genotype A.[3][4][5][6][7] Given that improvements in treatment response have been observed with interferon treatment and not nucleotide/nucleoside therapy, further research would be beneficial prior to recommending genotype testing in clinical practice and correlating with treatment response. Genotyping is not currently recommended for routine testing or follow-up of patients with chronic HBV infection.[2]
Worldwide distribution of genotypes varies.[8]
Genotype A is mainly found in the US, north-western Europe, and south-eastern Africa.
Genotype A is associated with a higher rate of HBeAg seroconversion with interferon therapy compared with genotypes B, C, and D.
Genotypes B and C are mainly found in Southeast Asia, China, and Japan.
Genotype B is associated with earlier age of HBeAg seroconversion, less active hepatic necro-inflammation, more sustained remission after HBeAg seroconversion, a slower rate of progression to cirrhosis, and a lower rate of hepatocellular carcinoma (HCC), compared with genotype C.
Genotype C is the genotype most associated with cirrhosis and HCC.
Genotype D is mainly found in the Mediterranean basin and some parts of Asia, but has been found worldwide.
Genotype E is mainly found in West and Central Africa.
Genotype F is mainly found in Central and South America.
Genotype G is mainly found in Mexico.
Genotype H is mainly found in Mexico.
Genotypes I and J have also been identified.
Use of this content is subject to our disclaimer