The clinical diagnostic approach to persistent pulmonary infiltrate necessitates an evaluation of host factors (age, comorbidities, immunodeficiency), the severity of symptoms, and the possibility of a non-infectious aetiology. History and clinical examinations, augmented by laboratory evaluation and radiographic techniques, can narrow the differential diagnosis.[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
[20]Low DE, Mazzulli T, Marrie T. Progressive and nonresolving pneumonia. Curr Opin Pulm Med. 2005 May;11(3):247-52.
http://www.ncbi.nlm.nih.gov/pubmed/15818188?tool=bestpractice.com
[21]Weyers CM, Leeper KV. Nonresolving pneumonia. Clin Chest Med. 2005 Mar;26(1):143-58.
http://www.ncbi.nlm.nih.gov/pubmed/15802176?tool=bestpractice.com
History
Fever and productive cough may indicate an infectious aetiology, but patients with persistent pulmonary infiltrate may be asymptomatic, with only radiological findings. Immunosuppressed patients must be evaluated initially for infection.
A history of pulmonary infection, especially with a pathogen that is associated with delayed radiological resolution, can be a harbinger of slowly resolving pneumonia.
Comorbid diseases (e.g., COPD, alcohol misuse, renal failure) may also delay radiological resolution of pneumonia.[4]Mittl RL Jr, Schwab RJ, Duchin JS, et al. Radiographic resolution of community-acquired pneumonia. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):630-5.
http://www.ncbi.nlm.nih.gov/pubmed/8118630?tool=bestpractice.com
[6]El Solh AA, Aquilina AT, Gunen H, et al. Radiographic resolution of community-acquired bacterial pneumonia in the elderly. J Am Geriatr Soc. 2004 Feb;52(2):224-9.
http://www.ncbi.nlm.nih.gov/pubmed/14728631?tool=bestpractice.com
Epidemiological exposure to pathogens, such as Mycobacterium tuberculosis, fungi (histoplasmosis, coccidioidomycosis, blastomycosis, and cryptococcosis), or parasites, should be evaluated.
[Figure caption and citation for the preceding image starts]: Radiographic resolution of causal pathogens of pneumoniaCreated by Athanasia Pataka [Citation ends].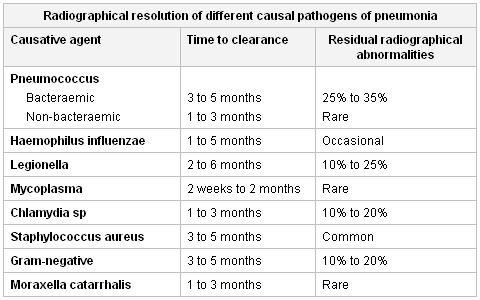 Non-infectious aetiologies (e.g., radiation, drug reactions, and diffuse alveolar haemorrhage) are responsible for 20% of cases of persistent pulmonary infiltrates and must be considered Iin the absence of an infectious cause.[12]Menendez R, Torres A, Zalacaín R, et al. Neumofail Group. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004 Nov;59(11):960-5.
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1746855&blobtype=pdf
http://www.ncbi.nlm.nih.gov/pubmed/15516472?tool=bestpractice.com
Non-infectious aetiologies (e.g., radiation, drug reactions, and diffuse alveolar haemorrhage) are responsible for 20% of cases of persistent pulmonary infiltrates and must be considered Iin the absence of an infectious cause.[12]Menendez R, Torres A, Zalacaín R, et al. Neumofail Group. Risk factors of treatment failure in community acquired pneumonia: implications for disease outcome. Thorax. 2004 Nov;59(11):960-5.
http://www.pubmedcentral.nih.gov/picrender.fcgi?artid=1746855&blobtype=pdf
http://www.ncbi.nlm.nih.gov/pubmed/15516472?tool=bestpractice.com
Malignancies, and endobronchial obstruction from these lesions, are a common cause of persistent pulmonary infiltrate and should always be considered in the differential diagnosis. Patients with a smoking history, haemoptysis, cachexia, or weight loss with non-resolving opacities should be evaluated for malignancy.
Haematuria may indicate alveolar haemorrhage syndromes, whereas joint pain or rashes indicate connective tissue disorders.
Asthma and persistent migratory infiltrate may suggest allergic bronchopneumonic aspergillosis.
Medication history is important, as drugs (e.g., amiodarone, bleomycin, cyclophosphamide, vincristine, taxanes) can cause pulmonary infiltrate.
Home, work environments (dust, allergens, pets), and recent travels should be assessed. Occupational and environmental exposures, radiation, and collagen-related diseases can also cause persistent pulmonary infiltrate.
Physical examination
The traditional pulmonary findings of lung disease (e.g., crackles and rales on auscultation, dullness to percussion over the chest) may be absent in the presence of persistent radiographic findings of pulmonary infiltrate. However, the examination of a patient with slowly resolving or non-resolving pneumonia should proceed in the same comprehensive manner as for any patient with a significant illness.
In immunocompetent patients with pneumonia, clinical response to antibiotic therapy is the most important determinant of further diagnostic studies for the assessment of persistent pulmonary infiltrate.[13]Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007 Oct;132(4):1348-55.
http://www.ncbi.nlm.nih.gov/pubmed/17934120?tool=bestpractice.com
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
Clinical improvement and resolution of leukocytosis support response to antibiotic therapy, even when chest radiographic abnormalities persist and observation alone is reasonable.[2]Kuru T, Lynch JP 3rd. Non-resolving or slowly resolving pneumonia. Clin Chest Med. 1999 Sep;20(3):623-51.
http://www.ncbi.nlm.nih.gov/pubmed/10516909?tool=bestpractice.com
When clinical improvement has not occurred and chest radiographic findings are unchanged or worse, or if there is a lack of even partial radiographic resolution by 4 weeks, further evaluation is essential, even in asymptomatic patients.[2]Kuru T, Lynch JP 3rd. Non-resolving or slowly resolving pneumonia. Clin Chest Med. 1999 Sep;20(3):623-51.
http://www.ncbi.nlm.nih.gov/pubmed/10516909?tool=bestpractice.com
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
In HIV-infected patients, skin manifestations of a pulmonary-associated bacterial, fungal, viral, or neoplastic disorder may be present (e.g., cryptococcosis, Kaposi's sarcoma). When a disseminated infection (fungal or mycobacterial) is suspected, an ophthalmological examination, including of the fundus and optic disc, should be performed to check for micro-haemorrhages.
Pulmonary congestion, peripheral oedema, and elevated jugular venous pressure suggest volume overload in patients with heart failure. Clubbing of the digits may indicate idiopathic pulmonary fibrosis, asbestosis, malignancies, and (rarely) sarcoidosis or hypersensitivity pneumonitis.[72]National Institute for Health and Care Excellence. Idiopathic pulmonary fibrosis in adults: diagnosis and management. May 2017 [internet publication].
https://www.nice.org.uk/guidance/cg163
[73]Spicknall KE, Zirwas MJ, English JC 3rd. Clubbing: an update on diagnosis, differential diagnosis, pathophysiology, and clinical relevance. J Am Acad Dermatol. 2005 Jun;52(6):1020-8.
http://www.ncbi.nlm.nih.gov/pubmed/15928621?tool=bestpractice.com
Involvement of extrapulmonary sites (eyes, joints, skin, kidneys, heart, salivary glands) may suggest the presence of systemic vasculitis, sarcoidosis, and connective tissue disorders.
Laboratory
The clinical response to previous antibiotic therapy is the most important indicator of further diagnostic studies for assessing persistent pulmonary infiltrate.[13]Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007 Oct;132(4):1348-55.
http://www.ncbi.nlm.nih.gov/pubmed/17934120?tool=bestpractice.com
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
In patients with non-resolving pneumonia, an initial microbiological result to confirm or deny the diagnosis of community-acquired pneumonia is essential, as the micro-organism itself may explain slower resolution (e.g., Legionella pneumonia, bacteraemic pneumonia). In patients with pneumonia, reduction of leukocytes and C-reactive protein strongly supports response to antibiotic therapy, even with persistent chest radiographic abnormalities, and further laboratory evaluation is not necessary initially.[2]Kuru T, Lynch JP 3rd. Non-resolving or slowly resolving pneumonia. Clin Chest Med. 1999 Sep;20(3):623-51.
http://www.ncbi.nlm.nih.gov/pubmed/10516909?tool=bestpractice.com
Microbiological investigation may consist of:[1]Menendez R, Perpina M, Torres A. Evaluation of non-resolving and progressive pneumonia. Semin Respir Infect. 2003 Jun;18(2):103-11.
http://www.ncbi.nlm.nih.gov/pubmed/12840791?tool=bestpractice.com
[13]Menendez R, Torres A. Treatment failure in community-acquired pneumonia. Chest. 2007 Oct;132(4):1348-55.
http://www.ncbi.nlm.nih.gov/pubmed/17934120?tool=bestpractice.com
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
[68]Centers for Disease Control and Prevention. Updated Guidelines for the Use of Nucleic Acid Amplification Tests in the Diagnosis of Tuberculosis. Jan 2009 [internet publication].
https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5801a3.htm
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
[74]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67.
https://www.doi.org/10.1164/rccm.201908-1581ST
http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
[75]Sordé R, Falcó V, Lowak M, et al. Current and potential usefulness of pneumococcal urinary antigen detection in hospitalized patients with community-acquired pneumonia to guide antimicrobial therapy. Arch Intern Med. 2011 Jan 24;171(2):166-72.
http://www.ncbi.nlm.nih.gov/pubmed/20876397?tool=bestpractice.com
[76]Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017 Jun;153(6):e129-46.[77]Martin-Loeches I, Torres A, Nagavci B, et al. ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Eur Respir J. 2023 Apr 3;61(4):2200735.
https://erj.ersjournals.com/content/61/4/2200735
http://www.ncbi.nlm.nih.gov/pubmed/37012080?tool=bestpractice.com
Sputum Gram stain
Sputum conventional bacterial culture
Direct immunofluorescence (for Legionella)
Influenza and respiratory syncytial virus (in winter months)
Sputum acid-fast bacillus smear and culture and sensitivity if tuberculosis (TB) is suspected
Nucleic acid amplification tests (should be performed on at least one respiratory specimen when a diagnosis of TB is being considered)
Stains for fungi in respiratory samples
Blood cultures
Urinary Legionella antigen (serotype 1) and Streptococcus pneumoniae antigen assays
Stains and cultures for bacteria and anaerobes in pleural fluid (if pleural effusion is present).
Multiplex polymerase chain reaction testing of lower respiratory tract samples (for viral and/or bacterial detection in patients requiring non-standard antibiotics).
Initial and follow-up serology for Legionella species and atypical pathogens may be necessary if polymerase chain reaction testing is not available.[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
D-dimer testing can be useful to rule out pulmonary embolism.[78]Kearon C, Ginsberg JS, Douketis J, et al. An evaluation of D-dimer in the diagnosis of pulmonary embolism: a randomized trial. Ann Intern Med. 2006 Jun 6;144(11):812-21.
http://www.ncbi.nlm.nih.gov/pubmed/16754923?tool=bestpractice.com
[79]Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998 Dec 15;129(12):997-1005.
http://www.ncbi.nlm.nih.gov/pubmed/9867786?tool=bestpractice.com
Auto-antibody testing (rheumatoid factor, antinuclear antibodies, and anti-neutrophil cytoplasmic autoantibodies) should be ordered if connective tissue disorders or vasculitis are suspected. Serum angiotensin-converting enzyme may be considered when sarcoidosis is suspected, although its utility is disputed.[80]Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007 Nov 22;357(21):2153-65.
http://content.nejm.org/cgi/content/full/357/21/2153
http://www.ncbi.nlm.nih.gov/pubmed/18032765?tool=bestpractice.com
Eosinophilia may suggest eosinophilic pneumonia.[81]Meyer KC. The role of bronchoalveolar lavage in interstitial lung disease. Clin Chest Med. 2004 Dec;25(4):637-49.
http://www.ncbi.nlm.nih.gov/pubmed/15564013?tool=bestpractice.com
Imaging
Chest x-ray findings dictate the timing and choice of further evaluation for persistent pulmonary infiltrate. In most instances, chest computed tomography (CT) is the most helpful radiographic modality. Infiltrations that persist without even partial resolution within 1 month, or those that fail to resolve within 12 weeks, should be further investigated even in asymptomatic patients.[6]El Solh AA, Aquilina AT, Gunen H, et al. Radiographic resolution of community-acquired bacterial pneumonia in the elderly. J Am Geriatr Soc. 2004 Feb;52(2):224-9.
http://www.ncbi.nlm.nih.gov/pubmed/14728631?tool=bestpractice.com
[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
Chest CT can detect pleural disease, complications of initial pneumonia (empyema or abscess), mediastinal abnormalities, and other non-infectious causes of persistent pulmonary infiltrate.[14]Woodhead M, Blasi F, Ewig S, Garau J, et al. Guidelines for the management of adult lower respiratory tract infections - full version. Clin Microbiol Infect. 2011 Nov;17 (Suppl 6):E1-59.
https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(14)61404-X/fulltext
http://www.ncbi.nlm.nih.gov/pubmed/21951385?tool=bestpractice.com
American Thoracic Society/Infectious Diseases Society of America guidelines do not recommend follow-up chest imaging in patients with community-acquired pneumonia whose symptoms are improving.[74]Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-67.
https://www.doi.org/10.1164/rccm.201908-1581ST
http://www.ncbi.nlm.nih.gov/pubmed/31573350?tool=bestpractice.com
Active interstitial pneumonitis may be indicated by the presence of ground-glass opacification on high-resolution chest CT, but it is not specific. CT is also useful for directing fibre-optic bronchoscopy. CT pulmonary angiography or V/Q scan is indicated if pulmonary embolism is suspected.[79]Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998 Dec 15;129(12):997-1005.
http://www.ncbi.nlm.nih.gov/pubmed/9867786?tool=bestpractice.com
Bronchoscopy
Fibre-optic bronchoscopy is indicated when diagnostic uncertainty remains after laboratory and radiographic evaluations are completed. Bronchoscopy has the advantage of minimal morbidity and is the preferred method of endoscopic investigation. It may be used as a diagnostic modality in approximately half of persistent pulmonary infiltrate cases.[16]Arancibia F, Ewig S, Martinez JA, et al. Antimicrobial treatment failures in patients with community-acquired pneumonia: causes and prognostic implications. Am J Respir Crit Care Med. 2000 Jul;162(1):154-60.
http://www.atsjournals.org/doi/full/10.1164/ajrccm.162.1.9907023
http://www.ncbi.nlm.nih.gov/pubmed/10903235?tool=bestpractice.com
[82]Feinsilver SH, Fein AM, Niederman MS, et al. Utility of fiberoptic bronchoscopy in nonresolving pneumonia. Chest. 1990 Dec;98(6):1322-6.
http://www.ncbi.nlm.nih.gov/pubmed/2245668?tool=bestpractice.com
Bronchoscopy allows direct observation of affected areas and collection of samples. Protected brush specimen (PBS), bronchoalveolar lavage (BAL), and bronchial or transbronchial biopsy can be used. Microbiological studies of BAL and PBS may include stains and cultures for the usual bacteria; specific stains and cultures for mycobacteria, Legionella, fungi, virus; and direct immunofluorescence forLegionella. The established cut-off point to differentiate between colonisation and infection is 10³ colony-forming units (cfu)/mL for PBS and 10⁴ cfu/mL for BAL fluid in immunocompetent patients, but previous antibiotic treatment should be considered. In intubated patients, endotracheal aspirates with quantitative cultures (>10⁶ cfu/mL) and sampling of distal airways (mini-BAL, protected telescoping catheter, or a blind bronchial suction sample) can be useful.
Transthoracic needle aspiration, thoracoscopic lung biopsy, or open-lung biopsy may be useful if bronchoscopy is unsuccessful or does not yield a definitive diagnosis. In patients with undiagnosed interstitial lung disease, transbronchial lung cryobiopsy has been suggested as an alternative to surgical lung biopsy if a tissue sample is required.[83]Korevaar DA, Colella S, Fally M, et al. European Respiratory Society guidelines on transbronchial lung cryobiopsy in the diagnosis of interstitial lung diseases. Eur Respir J. 2022 Nov 10;60(5):2200425.
https://erj.ersjournals.com/content/60/5/2200425
http://www.ncbi.nlm.nih.gov/pubmed/35710261?tool=bestpractice.com
 Non-infectious aetiologies (e.g., radiation, drug reactions, and diffuse alveolar haemorrhage) are responsible for 20% of cases of persistent pulmonary infiltrates and must be considered Iin the absence of an infectious cause.[12]
Non-infectious aetiologies (e.g., radiation, drug reactions, and diffuse alveolar haemorrhage) are responsible for 20% of cases of persistent pulmonary infiltrates and must be considered Iin the absence of an infectious cause.[12]