Recommendations
Urgent
Time is brain” - ischaemic stroke is an emergency.
Early initiation of reperfusion strategies (intravenous thrombolysis or mechanical thrombectomy) within 4.5 hours from onset of symptoms, if not contraindicated, is associated with improved functional outcomes.[63][64][65]
Manage any airway, breathing, and circulatory insufficiencies requiring urgent treatment. In particular:
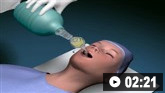
Consider endotracheal intubation for patients who are unable to protect their airway or for those presenting with a Glasgow Coma Scale score ≤8. This should be done by an anaesthetist or trained emergency department staff.[101]
Give supplemental oxygen only if oxygen saturation drops below 93%.[102] Although the National Institute for Health and Care Excellence (NICE) in the UK recommends starting oxygen only if oxygen saturation drops below 95%, latest evidence suggests that in patients with stroke there are no benefits to initiating oxygen therapy when SpO₂ is ≥93%, and it may cause harm.[67][102]
Monitor controlled oxygen therapy. An upper SpO 2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. Evidence suggests that liberal use of supplemental oxygen (target SpO 2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[103] A lower target SpO 2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[104]
Do not routinely give oxygen to people who are not hypoxic.[67]
Assess the patient’s level of consciousness using the Glasgow Coma Scale. In patients with decreased level of consciousness or coma, urgently exclude haemorrhage and stroke mimics such as seizures.
Admit everyone with suspected stroke directly to a hyperacute (or acute) stroke unit as soon as possible; UK guidelines recommend doing this within 4 hours of presentation to hospital.[67]
Intravenous thrombolysis should be given (if not contraindicated) if treatment is started as soon as possible within 4.5 hours of onset of symptoms, AND if intracranial haemorrhage has been excluded by imaging.[65][67]
Patients with acute ischaemic stroke, regardless of age or stroke severity, who were last known to be well more than 4.5 hours earlier should also be considered for thrombolysis in selected circumstances - see Further information.[68]
Do not delay intravenous thrombolysis (if indicated) while waiting for results or waiting to perform tests, unless you suspect contraindications that must be ruled out first (eg., hypoglycaemia, coagulopathy), or while monitoring for further improvement.[70][71] Reperfusion (via thrombolysis, thrombectomy or both) is a time-critical intervention.
Exclude hypoglycaemia and hyperglycaemia before giving thrombolysis; hypoglycaemia is a stroke mimic and hyperglycaemia is associated with intracerebral bleeding and worse clinical outcomes.[67][69]
The 2023 National Clinical Guideline for Stroke for the UK and Ireland recommends to consider thrombolysis with either alteplase or tenecteplase in patients with acute ischaemic stroke within 4.5 hours of known onset.[68] The use of intravenous tenecteplase is off-label for this indication in the UK.
In the community: arrange immediate emergency admission to an hyperacute (or acute, depending on availability) stroke unit for anyone with:
Persisting neurological symptoms who is suspected of having acute stroke (or emergent transient ischaemic attack [TIA])[67][68]
Resolved neurological symptoms who has a bleeding disorder or is taking an anticoagulant because haemorrhage must be excluded.[68]
In people with suspected TIA, follow the recommendations in Transient ischaemic attack.
Key Recommendations
Monitor temperature and maintain normal body physiology.[68][105]
Give an antipyretic (e.g., paracetamol) in patients with high temperature.
Do not use therapeutic hypothermia (i.e., active cooling) to reduce the risk of secondary brain damage.[67]
Maintain blood glucose concentration between 4 and 11 mmol/L.[67]
Give intravenous insulin and glucose to all adults with type 1 diabetes with threatened or actual stroke. Follow your local protocol.[67]
Give antihypertensive treatment only if there is a hypertensive emergency with one or more of the following serious concomitant conditions [67]
Hypertensive encephalopathy
Hypertensive nephropathy
Hypertensive cardiac failure/myocardial infarction
Aortic dissection
Pre-eclampsia/eclampsia.
In people who would be eligible for secondary prevention treatment for atrial fibrillation or flutter:[68]
Perform prolonged ECG monitoring (at least 24 hours)
Consider prolonged sequential or continuous ECG monitoring with an external patch, wearable recorder, or implantable loop recorder in those in whom no other cause of stroke has been found, particularly if they have a pattern of cerebral ischaemia on brain imaging suggestive of cardioembolism.
Monitor the patient for signs of elevated intracranial pressure (ICP). Repeat the computed tomography (CT) head immediately if you suspect elevated ICP which may present as:
A reducing level of consciousness
Severe headache
Nausea/vomiting
A sudden increase in blood pressure.
Refer immediately to a neurosurgeon any patients with large middle cerebral artery territory infarcts and those with large infarctions affecting the cerebellum.[68][69] These types of stroke have a very high mortality if urgent neurosurgical intervention is delayed.[69]
Consult immediately with a neurologist or neurosurgeon if the patient has uncontrolled or recurrent seizures, or status epilepticus. The choice of anticonvulsant will depend on the patient characteristics.[69] See Status epilepticus.
Follow your hospital protocol. Levetiracetam and sodium valproate are commonly used.
Mechanical thrombectomy should be performed:[68]
As soon as possible and within 6 hours of symptom onset (together with intravenous thrombolysis, if not contraindicated and within the licensed time window) in patients who have confirmed occlusion of the proximal anterior circulation.
As soon as possible in patients who were last known to be well between 6 and 24 hours previously (including wake-up strokes) and with no previous disability (modified Rankin Scale [mRS] 0 or 1) combined with thrombolysis, if eligible:
Those who have confirmed occlusion of the proximal anterior circulation (ICA and/or M1) causing a disabling neurological deficit (National Institutes of Health Stroke Scale [NIHSS] score of 6 or more). [ NIH Stroke Score Opens in new window ]
If there is potential to salvage brain tissue, as shown by perfusion imaging.
Between 6 and 12 hours: an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) score of 3 or more, irrespective of the core infarct size.
Between 12 and 24 hours: an ASPECTS score of 3 or more and CT or MRI perfusion mismatch of greater than 15 mL, irrespective of the core infarct size.
Thrombectomy should be considered together with thrombolysis (if not contraindicated and within the licensed time window) as soon as possible for patients known to be well up to 24 hours previously (including wake-up strokes):[67]
Who have confirmed occlusion of the proximal posterior circulation (i.e., basilar or posterior cerebral artery)
AND
If there is potential to salvage brain tissue, as shown by perfusion imaging.
Caution should be exercised when considering mechanical thrombectomy for patients presenting between 12 and 24 hours of onset and/or over the age of 80 owing to the paucity of data in these groups.[68]
Candidates for thrombectomy alone include patients who cannot receive thrombolysis (e.g., with contraindications including recent surgery, current anticoagulation use).[68]
Offer aspirin (or an alternative antiplatelet agent if the patient is allergic to or intolerant of aspirin) as soon as possible and definitely within 24 hours to all patients in whom intracerebral haemorrhage has been excluded by imaging.[67]
Do not use anticoagulation treatment routinely to treat acute stroke.[67]
Do not start statin treatment immediately. Usually it would be appropriate to start statin treatment once a patient can swallow medication safely, and there is consensus that it is safe to start statins after 48 hours.[67][68]
Offer high-intensity statin therapy (unless contraindicated or investigation confirms no evidence of atherosclerosis).[68]
Continue statin treatment in people who are already receiving statins.[67]
Consider increasing the statin intensity or dose if the patient is not currently taking a high-intensity statin at the maximum tolerated dose.[61]
“Time is brain” - ischaemic stroke is an emergency.
Early initiation of reperfusion strategies (intravenous thrombolysis or mechanical thrombectomy) within 4.5 hours from onset of symptoms, if not contraindicated, is associated with improved functional outcomes.[63][64]
The goals of treatment are to:
Restore blood flow
Support energy metabolism in ischaemic tissue
Treat complications of stroke-related oedema, such as elevated intracranial pressure, and prevent common acute complications such as those associated with acute reperfusion therapy (e.g., orolingual angioedema secondary to alteplase). See Complications.
People with disabling ischaemic stroke are candidates for hyperacute treatment with thrombolysis, mechanical thrombectomy, or both.
For those with non-disabling ischaemic stroke (i.e., minor stroke [NIHSS ≤3]) see Non-urgent initial management, below.
Stabilisation
Manage any airway, breathing, and circulatory insufficiencies requiring urgent treatment. In particular:
Consider endotracheal intubation for patients who are unable to protect their airway or for those presenting with a depressed level of consciousness (Glasgow Coma Scale score ≤8). This should be done by an anaesthetist or trained emergency department staff.[101]
Give supplemental oxygen only if oxygen saturation drops below 93%.[102] Although the National Institute for Health and Care Excellence (NICE) in the UK recommends starting oxygen only if oxygen saturation drops below 95%, latest evidence suggests that in patients with stroke there are no benefits to initiating oxygen therapy when SpO₂ is ≥93%, and it may cause harm.[67][102]
Monitor controlled oxygen therapy. An upper SpO 2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. Evidence suggests that liberal use of supplemental oxygen (target SpO 2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[103] A lower target SpO 2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[104]
Do not routinely give oxygen to people who are not hypoxic.[67]
Evidence: Target oxygen saturation in acutely ill adults
Too much supplemental oxygen increases mortality.
Evidence from a large systematic review and meta-analysis supports conservative/controlled oxygen therapy versus liberal oxygen therapy in non-hypercapnic acutely ill adults.
Guidelines differ in their recommendations on target oxygen saturation in acutely unwell adults who are receiving supplemental oxygen.
The 2017 British Thoracic Society (BTS) guideline recommends a target SpO₂ range of 94% to 98% for patients not at risk of hypercapnia, whereas the 2022 Thoracic Society of Australia and New Zealand (TSANZ) guideline recommends 92% to 96%.[104][107]
The Global Initiative For Asthma (GINA) guidelines recommend a target SpO 2 range of 93% to 96% in the context of severe exacerbations of asthma.[108]
A systematic review including a meta-analysis of data from 25 randomised controlled trials published in 2018 found that in adults with acute illness, liberal oxygen therapy (broadly equivalent to a target saturation >96%) is associated with higher mortality than conservative oxygen therapy (broadly equivalent to a target saturation ≤96%).[103] In-hospital mortality was 11 per 1000 higher for the liberal oxygen therapy group versus the conservative therapy group (95% CI 2 to 22 per 1000 more). Mortality at 30 days was also higher in the group who had received liberal oxygen (RR 1.14, 95% CI 1.01 to 1.29). The trials included adults with sepsis, critical illness, stroke, trauma, myocardial infarction, and cardiac arrest, and patients who had emergency surgery. Studies that were limited to people with chronic respiratory illness or psychiatric illness, and patients on extracorporeal life support, receiving hyperbaric oxygen therapy, or having elective surgery, were all excluded from the review.
An upper SpO₂ limit of 96% is therefore reasonable when administering supplemental oxygen to patients with acute illness who are not at risk of hypercapnia. However, a higher target may be appropriate for some specific conditions (e.g., pneumothorax, carbon monoxide poisoning, cluster headache, and sickle cell crisis).[102]
In 2019 the BTS reviewed its guidance in response to this systematic review and meta-analysis and decided an interim update was not required.[109]
The committee noted that the systematic review supported the use of controlled oxygen therapy to a target.
While the systematic review showed an association between higher oxygen saturations and higher mortality, the BTS committee felt the review was not definitive on what the optimal target range should be. The suggested range of 94% to 96% in the review was based on the lower 95% confidence interval and the median baseline SpO 2 from the liberal oxygen groups, along with the earlier 2015 TSANZ guideline recommendation.
Subsequently, experience during the COVID-19 pandemic has also made clinicians more aware of the feasibility of permissive hypoxaemia.[110]
Management of oxygen therapy in patients in intensive care is specialised and informed by further evidence (not covered in this summary) that is more specific to this setting.[111][112][113]
Refer to hyperacute or acute stroke unit
In hospital
Admit everyone with suspected stroke directly to a hyperacute (or acute, depending on availability) stroke unit as soon as possible; UK guidelines recommend doing this within 4 hours of presentation to hospital.[67]
This is to begin thrombolysis as quickly as possible (if indicated) and to help prevent complications.[67][68]
Evidence: Hyperacute stroke units
People who have had a stroke are more likely to be alive, independent, and living at home at 1 year post-stroke if they received care in an acute inpatient stroke unit, compared with less-organised alternative care. Care in a dedicated stroke ward seems to be the most effective approach.
The 2016 UK Royal College of Physicians national clinical guideline on stroke, subsequently updated in 2023 (the National Clinical Guideline for Stroke for the UK and Ireland), cited a 2013 Cochrane review by the Stroke Unit Trialists’ Collaboration to support its recommendation to admit patients with suspected acute stroke to a hyperacute stroke unit.[68]
This Cochrane review (search date January 2013) assessed the effect of organised inpatient stroke unit care compared with alternative less-organised forms of care for people with acute stroke.[114]
It included 28 randomised controlled trials (RCTs), involving a total of 5855 patients admitted to hospital with stroke (using a clinical definition of stroke: focal neurological deficit due to cerebrovascular disease, excluding subarachnoid haemorrhage and subdural haematoma).
Results for organised stroke unit care versus alternative care (general wards or mixed rehabilitation units) showed that patients in stroke units had a reduced “risk of death, “death or institutionalised care”, and “death or dependency” at final follow-up (median 1 year).
These conclusions were confirmed in a more recent version (2020) of this Cochrane review, which included 29 RCTs (n=5902), new outcome data from recent trials, and further assessment via network meta-analysis.[115]
[  ]
]
In the community
Arrange immediate emergency admission to an hyperacute (or acute, depending on availability) stroke unit for anyone with:
Persisting neurological symptoms who is suspected of having acute stroke (or emergent transient ischaemic attack)[67][68]
Resolved neurological symptoms who has a bleeding disorder or is taking an anticoagulant because haemorrhage must be excluded.[68]
The European Stroke Organisation (ESO) has issued a weak recommendation for tenecteplase over alteplase for patients within 4.5 hours of ischaemic stroke and pre-hospital management with a mobile stroke unit in order to increase the rate of early reperfusion and to shorten the time from imaging to treatment initiation.[106] The use of intravenous tenecteplase is off-label for this indication in the UK.
Thrombolysis
Follow your local protocols for recommendations on intravenous thrombolysis.
Guidelines in the UK and from the European Stroke Organisation (ESO) recommend to give intravenous alteplase (recombinant tissue plasminogen activator) to eligible patients if not contraindicated and:[65][67][68][116]
Treatment is started as soon as possible within 4.5 hours of onset of stroke symptoms
AND
Intracranial haemorrhage has been excluded using appropriate imaging techniques.
Recent guidelines recommend to consider thrombolysis with tenecteplase as an equal alternative to alteplase in these patients but tenecteplase is off-label for this indication in the UK.[65][68]
The National Clinical Guideline for Stroke for the UK and Ireland recommends patients with acute ischaemic stroke, regardless of age or stroke severity, who were last known to be well more than 4.5 hours earlier should be considered for thrombolysis with alteplase if intracranial haemorrhage has been excluded and:[68]
Treatment can be started between 4.5 and 9 hours of known onset, or within 9 hours of the midpoint of sleep when they have woken with symptoms
AND
There is evidence of the potential to salvage brain tissue on CT perfusion or MRI (DWI-FLAIR mismatch [diffusion-weighted MRI and fluid-attenuated inversion recovery MRI mismatch]).
This should be irrespective of whether they have a large artery occlusion and require mechanical thrombectomy.[68]
The ESO further recommends intravenous thrombolysis for:[65]
Patients who were last seen well 4.5 to 9 hours earlier (known onset time), with CT or MRI core/perfusion mismatch, and for whom mechanical thrombectomy is either not indicated or not planned
Patients with acute ischaemic stroke on awakening from sleep, who were last seen well more than 4.5 hours earlier, who have MRI DWI-FLAIR mismatch, and for whom mechanical thrombectomy is either not indicated or not planned.
Refer to the prescribing information for contraindications to thrombolysis with alteplase. Examples include recent surgery and current anticoagulation use.
Do not delay treatment with alteplase while waiting for results or waiting to perform tests, unless you suspect contraindications that must be ruled out first (eg., hypoglycaemia, coagulopathy), or while monitoring for further improvement.[70][71]
Consider reducing blood pressure to 185/110 mmHg or lower in people who are candidates for intravenous thrombolysis.[67][117]
More info: Choice of thrombolytic agent
Alteplase is currently the only approved fibrinolytic treatment for patients with acute ischaemic stroke. The use of intravenous tenecteplase is off-label for this indication in the UK. However, the 2023 National Clinical Guideline for Stroke for the UK and Ireland recommends to consider thrombolysis with either alteplase or tenecteplase in all patients with acute ischaemic stroke within 4.5 hours of known onset.[68]
In 2023, the European Stroke Organisation (ESO) issued a strong recommendation to consider tenecteplase as a safe and effective alternative to alteplase within 4.5 hours of ischaemic stroke due to large vessel occlusion based on meta-analysis of several randomised controlled trials (RCTs).[106][118][119][120][121][122] Tenecteplase is not recommended for patients with ischaemic stroke on awakening from sleep or of unknown onset who undergo no brain imaging other than CT.[68][106] The ESO cites a phase 3 RCT of patients with wake-up stroke selected with non-contrast CT. Treatment with tenecteplase was not associated with better functional outcome at 90 days versus the control group in patients selected with non-contrast CT imaging alone, and there was no difference in mortality between groups.[123] Intravenous thrombolysis should not delay mechanical thrombectomy.[106]
Evidence: Thrombolysis with alteplase within 4.5 hours of symptom onset
Alteplase improves functional outcomes after acute ischaemic stroke if given within 4.5 hours of onset of symptoms. There is an increased risk of intracranial haemorrhage with alteplase but this does not seem to affect death or dependency at 3 months.
A Cochrane systematic review (published 2014) assessed thrombolysis compared with placebo for patients with acute ischaemic stroke. It included 27 trials (n=10,187); most of the evidence came from the use of alteplase (12 trials; n=7012).[63]
There was a significant reduction in death or dependency by the end of follow-up when alteplase was given up to 6 hours from onset (8 randomised controlled trials [RCTs]; n=6729; OR 0.84, 95% CI 0.77 to 0.93; significant heterogeneity)
However, treatment within 3 hours was substantially more beneficial and there was no heterogeneity (6 RCTs; n=1779; OR 0.65 95% CI 0.54 to 0.80).
In 2012 the UK National Institute for Health and Care Excellence (NICE) updated their guidance on alteplase, following a technical appraisal, to extend the window for treatment from 3 hours to up to 4.5 hours after the onset of symptoms.[116] This window for treatment is also supported by the National Clinical Guideline for Stroke for the UK and Ireland.[68]
The decision by NICE was based mainly on the third European Cooperative Acute Stroke Study (ECASS III).[124]
821 people with acute ischaemic stroke were randomised to receive alteplase or placebo.
Alteplase increased the proportion of people with a favourable outcome (modified Rankin Scale [mRS] score 0-1; 52.4% with alteplase vs. 45.2% with placebo; RR 1.16, 95% CI 1.01 to 1.34).
There was an increased risk of intracranial haemorrhage with alteplase (27.0% vs. 17.6%, P=0.001) including symptomatic intracranial haemorrhage (2.4% with alteplase vs. 0.3% with placebo; RR 4.82, 95% CI 1.06 to 21.87).
There was no statistically significant difference in other serious adverse events or in all-cause mortality at 3 months (7.7% with alteplase vs. 8.4% with placebo, P=0.68).
In an additional analysis for NICE by the Evidence Review Group there was no significant difference in death or dependency at 90 days (RR 0.87, 95% CI 0.73 to 1.05), although there was a trend towards a better outcome with alteplase.[125]
NICE noted in its technical appraisal that the manufacturer had excluded the third International Stroke Trial (IST-3) in its submission because it included data that went beyond the then current UK market authorisation of alteplase.
Evidence: Thrombolysis with alteplase over 4.5 hours of symptom onset
In 2023 the National Clinical Guideline for Stroke for the UK and Ireland updated the guidance on alteplase to extend the window for treatment from 4.5 hours to up to 9 hours after the onset of symptoms, or within 9 hours of the midpoint of sleep if patients also have imaging evidence of the potential to salvage brain tissue.[68] Evidence underpinning this recommendation came from two sources: a large randomised controlled trial (RCT) and an individual patient data meta-analysis.[126][127]
The WAKE-UP trial
This was a multicentre (70 centres; 8 European countries) RCT comparing MRI-guided intravenous alteplase with placebo in people (aged 18-80) following an acute ischaemic stroke with unknown time of onset. In the trial, 503 people were randomised (alteplase [n=254] or placebo [n=249]).
Symptom onset was beyond 4.5 hours and included those who woke up with stroke symptoms or could not identify symptom onset and had an ischaemic lesion that was visible on MRI diffusion-weighted imaging but no parenchymal hyperintensity on fluid-attenuated inversion recovery (FLAIR) (indicating that the stroke had occurred approximately within the previous 4.5 hours).
People who had an area of infarction greater than one third of the middle cerebral artery territory, planned thrombectomy, or a NIHSS score >25 were excluded.
The trial found a favourable outcome (defined as a score of 0 or 1 on the modified Rankin scale [mRS] at 90 days) in 53.3% in the alteplase group versus 41.8% in the placebo group (adjusted odds ratio [OR] 1.61, 95% CI 1.09 to 2.36; P=0.02; NNT=9).
4.1% in the alteplase group died versus 1.2% in the placebo group (OR 3.38, 95% CI 0.92 to 12.52; P=0.07).
The rate of symptomatic intracranial haemorrhage was 2% in the alteplase group versus 0.4% in the placebo group (OR 4.95, 95% CI 0.57 to 42.87; P=0.15).
WAKE-UP was terminated early on the basis of cessation of funding.
Meta-analysis of individual patient data
The second source of evidence underpinning the National Clinical Guideline for Stroke for the UK and Ireland guideline for stroke recommendation on this issue was a meta-analysis to support the use of perfusion imaging to extend the time window for thrombolysis treatment.[68][127] Investigators conducted a systematic review and individual participant data meta-analysis of three trials: EXTEND, ECASS-4, and a subset of the EPITHET trial.[128][129][130]
211 patients in the alteplase group and 199 patients in the placebo group with mRS assessment data at 3 months were included in the analysis of the primary outcome.
More patients with CT or MR perfusion imaging-defined penumbra between 4.5 and 9 hours after onset or with wake-up stroke had a beneficial outcome with intravenous alteplase compared with placebo (7% absolute increase in excellent outcome at 3 months).
Symptomatic intracerebral haemorrhage was more common in the alteplase group (5%) versus the placebo group (<1%) (adjusted OR 9.7, 95% CI 1.23 to 76.55; P=0.031), but mortality did not differ between the groups (14% vs. 9%; OR 1.55, 95% CI 0.81 to 2.96; P=0.19).
Cochrane systematic review
A 2014 Cochrane systematic review (not cited as supporting this recommendation in the National Clinical Guideline for Stroke for the UK and Ireland) found, in selected patients with acute ischaemic wake‐up stroke, intravenous thrombolytic treatment of large vessel occlusion improved functional outcome without increasing the risk of death (similar improved outcomes were seen with endovascular thrombectomy). However, a possible increased risk of symptomatic intracranial haemorrhage associated with thrombolytic treatment could not be ruled out.[131]
The effect of intravenous thrombolytic treatment in wake‐up stroke in the meta‐analysis was moderate (risk ratio 1.13, 95% CI 1.01 to 1.26) and lower than the effect seen in the WAKE‐UP trial, which contributed the largest number of participants.
The reviewers highlighted the issue that four of the included trials were terminated early, including the WAKE UP trial, which is a potential source of bias.
In the UK, alteplase should only be administered within a well-organised stroke service by staff trained in delivering thrombolysis and in monitoring for complications.[67][68] Trained staff in emergency departments can also administer alteplase provided that the patient can be managed in an acute stroke service.[67]
Patients who are over 80 years old with mild or severe stroke also benefit from treatment with thrombolysis.[68]
Mechanical thrombectomy
The decision to offer mechanical thrombectomy (endovascular intervention) should be made by clinicians experienced in the use of thrombolysis for stroke and in interpretation of relevant imaging.[67] The procedure should only be carried out by appropriately trained specialists with regular experience in intracranial endovascular interventions, with appropriate facilities and neuroscience support.[85]
Patients eligible for mechanical thrombectomy should receive prior intravenous thrombolysis as rapidly as possible (unless contraindicated), irrespective of whether they have presented to an acute stroke centre or a thrombectomy centre.[68]
Candidates for thrombectomy alone include those who cannot receive thrombolysis (e.g., with contraindications including recent surgery, current anticoagulation use).[68]
Guidelines in the UK recommend for people with acute ischaemic stroke:[68]
Offering mechanical thrombectomy:
As soon as possible and within 6 hours of symptom onset (together with intravenous thrombolysis, if not contraindicated and within the licensed time window) to patients with no previous disability (modified Rankin Scale [mRS] 0 or 1) with confirmed occlusion of the proximal anterior circulation and proximal intracranial large artery occlusion causing a disabling neurological deficit (NIHSS score of 6 or more). [ NIH Stroke Score Opens in new window ]
As soon as possible to patients who were last known to be well between 6 and 24 hours previously (including wake-up strokes) and no previous disability (modified Rankin Scale [mRS] 0 or 1) combined with thrombolysis, if eligible:
Who have confirmed proximal intracranial large artery occlusion (ICA and/or M1) causing a disabling neurological deficit (NIHSS score of 6 or more) [ NIH Stroke Score Opens in new window ]
AND
If there is potential to salvage brain tissue, as shown by perfusion imaging:
Between 6 and 12 hours: an ASPECTS score of 3 or more, irrespective of the core infarct size
Between 12 and 24 hours: an ASPECTS score of 3 or more and CT or MRI perfusion mismatch of greater than 15 mL, irrespective of the core infarct size.
Considering thrombectomy together with intravenous thrombolysis (where not contraindicated and within the licensed time window) as soon as possible for patients known to be well up to 24 hours previously (including wake-up strokes):
Who have confirmed occlusion of the proximal posterior circulation (i.e., basilar or posterior cerebral artery)
AND
If there is potential to salvage brain tissue, as shown by perfusion imaging.
Caution should be exercised when considering mechanical thrombectomy for patients presenting between 12 and 24 hours of onset and/or over the age of 80 owing to the paucity of data in these groups.[68]
Evidence: Mechanical thrombectomy in anterior circulation stroke
Mechanical thrombectomy is an effective acute stroke treatment for selected patients with proximal large artery occlusions as an adjunct to intravenous thrombolysis, and for those patients with contraindications to intravenous thrombolysis but not to mechanical thrombectomy (e.g., recent surgery, anticoagulant use).
Mechanical thrombectomy within 6 hours
Due to the development of new neurointerventional techniques, numerous multicentre randomised controlled trials (RCTs) in this area have been published. In its evidence review for mechanical thrombectomy within 6 hours, the National Clinical Guideline for Stroke for the UK and Ireland cites a 2016 individual patient meta-analysis of five trials involving 1287 patients (634 assigned to endovascular thrombectomy, 653 assigned to standard care), which found endovascular therapy showed significant improvements in functional outcomes at 90 days.[68][132]
These RCTs (REVASCAT, MR CLEAN, EXTEND-IA, ESCAPE, and SWIFT-PRIME) evaluated the effects of endovascular treatment in addition to thrombolysis, compared with standard treatment (intravenous thrombolysis alone administered within 4.5 hours) in ‘early-presenting’ patients (typically within 6 hours) with proven large artery occlusion stroke. The trials only included patients with pre-stroke modified Rankin Scale (mRS) of 2 or less.[133][134][135][136][137]
Meta-analysis of individual patient data from these five trials by the HERMES collaboration concluded:[132]
Mechanical thrombectomy increases the odds of being in a less disabled category at 90 days (one point difference on the mRS) by more than twofold (odds ratio [OR] 2.26, 95% CI 1.67 to 3.06; P <0.0001).[138] The number needed to treat for one additional patient to have reduced disability of at least one point on the mRS with endovascular thrombectomy versus control was 3 (rounded up to whole number) (adjusted common odds ratio [cOR] 2.49, 95% CI 1.76 to 3.53; P <0.0001).
The trials were heterogenous in their patient selection (age, NIHSS score); imaging criteria (in particular whether the identification of salvageable brain tissue on neuroimaging was a trial inclusion criterion or not); inclusion of patients for whom intravenous thrombolysis was contraindicated; and timing of onset to endovascular treatment (from a maximum of 6 up to 12 hours).
The researchers took into account the heterogeneity in the studies in their chosen statistical methodology and adjusted for differences in subgroup analysis.
All the trials with an extended time window required imaging identification of the potential to salvage brain tissue prior to randomisation.
One opinion piece on the meta-analysis points out that outcomes in the control groups from the five individual trials were markedly different (mortality ranged from 12% to 22%; excellent functional recovery [defined by mRS scores of 0-1] ranged from 6% to 28%).[139]
Following the presentation of results of MR CLEAN, which showed a benefit of thrombectomy, most other trials stopped recruitment early (EXTEND IA, SWIFT PRIME, REVASCAT, ESCAPE). Some trials conducted an interim analysis; others acknowledged they were underpowered.[67] A further two trials (THRACE, THERAPY) were stopped prematurely after interim review triggered by the results from the HERMES trials.[67][140][141]
Mechanical thrombectomy beyond 6 hours
In its evidence review for thrombectomy beyond 6 hours, the National Clinical Guideline for Stroke for the UK and Ireland cites two RCTs of patients with a large ischaemic core who have previously been ineligible for trials of thrombectomy beyond 6 hours.[68] Both trials were stopped early for efficacy.
SELECT2 (n=352)[142]
This trial assigned patients in a 1:1 ratio to endovascular thrombectomy plus medical care or to medical care alone. There was significant benefit from mechanical thrombectomy plus medical care (compared with medical care alone) up to 24 hours after onset in patients aged 18-85 years with a pre-stroke mRS score of 0 or 1 who presented with a proximal large artery occlusion and an ASPECTS score of 3-5 or an infarct core of >50 mL.
Approximately 20% of patients treated with thrombectomy plus medical care reached functional independence (mRS 0-2) compared with 7% in those allocated to medical care alone (relative risk [RR] 2.97, 95% CI 1.60 to 5.51). The National Clinical Guideline for Stroke for the UK and Ireland summary of these results cites an NNT=8 for this outcome.[68]
Mortality was similar in the two groups but endovascular thrombectomy was associated with vascular complications.
ANGEL-ASPECT (n=456)[143]
This trial also demonstrated significant benefit from mechanical thrombectomy compared with medical management alone in patients aged 18-80 years with a pre-stroke mRS score of 0 or 1 and an internal carotid artery/M1 segment of the middle cerebral artery (ICA/M1) occlusion and either ASPECTS 3-5 or an infarct core of 70-100 mL presenting within 24 hours of onset.
The National Clinical Guideline for Stroke for the UK and Ireland summary of these results cites that thrombectomy increased the proportion of patients with functional independence (mRS 0-2) to 30% compared with 11.6% in the group having medical care alone with an NNT=6 for this outcome.[68]
However, thrombectomy in patients with large cerebral infarctions was associated with a higher proportion of intracranial haemorrhages (n=113 [49.1%]) compared with medical care alone (n=39 [17.3%]).
The National Clinical Guideline for Stroke for the UK and Ireland concludes that SELECT2, ANGEL-ASPECT, and DEFUSE-3 (which assessed thrombectomy at 6-16 hours with selection by perfusion imaging) provide robust evidence to select individuals for mechanical thrombectomy based on perfusion imaging (particularly perfusion mismatch >15 mL) in patients presenting between 12 and 24 hours after onset.[68][86][142][143]
Evidence: Mechanical thrombectomy in people with posterior circulation stroke
The National Clinical Guideline for Stroke for the UK and Ireland recommends that patients with acute ischaemic stroke in the posterior circulation within 12 hours of onset should be considered for mechanical thrombectomy (combined with thrombolysis if eligible) if they have a confirmed intracranial vertebral or basilar artery occlusion and their NIHSS score is 10 or more, combined with a favourable PC-ASPECTS score and PonsMidbrain Index. Caution should be exercised when considering mechanical thrombectomy for patients presenting between 12 and 24 hours of onset and/or over the age of 80 owing to the paucity of data in these groups.[68]
The guideline cites four randomised controlled trials (RCTs) to support its recommendations.
BASICS[144]
The BASICS study, a multicentre, open-label RCT, compared endovascular therapy with standard medical care in patients (n=300) within 6 hours of a stroke due to basilar artery occlusion.
A favourable functional outcome occurred in 68 of 154 patients (44.2%) in the endovascular group and 55 of 146 patients (37.7%) in the medical care group (risk ratio 1.18, 95% CI 0.92 to 1.50).
Although endovascular therapy and medical therapy did not differ significantly with respect to a favourable functional outcome, the researchers state that wide confidence intervals for the primary outcome suggested a substantial benefit of endovascular therapy could not be excluded.
The study may have been biased by non-consecutive enrolment and treatment of one third of eligible patients outside the trial.
BEST[145]
The BEST study, a multicentre, open-label RCT in China, compared endovascular therapy plus standard care with standard care alone in patients within 8 hours of a vertebrobasilar occlusion.
It found no difference between thrombectomy and standard care for functional outcomes in its intention to treat analysis. In the thrombectomy group 28/66 (42%) had a modified Rankin Scale (mRS) score 0-3 at 90 days versus 21/65 (32%) in the standard care group (adjusted odds ratio [OR] 1.74, 95% CI 0.81 to 3.74). It found no difference in 90-day mortality between groups (22/66 [33%] with thrombectomy vs. 25/65 [38%] with standard care; P=0.54).
However, the study may have been underpowered and it was stopped early due to the large number of crossovers between groups and poor recruitment (terminated after 131 patients had been randomly assigned).
BAOCHE[146]
The BAOCHE trial, conducted in Chinese patients, randomised 217 patients aged ≤80 years between 6 and 24 hours after basilar artery occlusion stroke onset with an NIHSS of 6 or more, a PC-ASPECTS score of 6 or more, or a Pons-Midbrain Index of 2 or more to receive thrombectomy plus medical therapy or medical therapy alone. It enrolled 82 patients (38%) in the 12-24 hour window.
It found an mRS of 0-3 occurred in 51 patients (46%) in the thrombectomy group and in 26 patients (24%) in the control group (adjusted rate ratio 1.81, 95% CI 1.26 to 2.60; P<0.001).
However, thrombectomy in these patients was associated with procedural complications and more cases of intracerebral haemorrhage compared with medical therapy alone.
There was no statistically significant difference between the two groups in mortality or symptomatic intracerebral haemorrhage (6/102 patients [6%] in the thrombectomy group and 1/88 patients [1%] in the control group [risk ratio 5.18, 95% CI 0.64 to 42.18]).
However, because patients in this trial included patients with basilar artery occlusion presenting within the time window of 6-24 hours, patients in the ‘best medical treatment’ group most likely did not receive intravenous thrombolysis. In contrast, in the BEST and BASIC trials, intravenous thrombolysis was used in 32% and 79.5% of patients, respectively. This factor might have contributed to the notable difference in results between trials.[147]
ATTENTION[148]
The ATTENTION trial was an RCT of endovascular thrombectomy versus best medical care for basilar artery occlusion across 36 centres in China.
It randomised 340 patients to either thrombectomy and best medical therapy or best medical therapy alone, with additional eligibility criteria of an NIHSS score of 10 or more, a PC-ASPECTS score of 6 or more, and in a time window of up to 12 hours after stroke onset. Patients over 80 years of age additionally had to have a PC-ASPECTS score of 8 or more and a pre-stroke mRS of 0-1.
Approximately one third of patients received intravenous thrombolysis.
Good functional status at 90 days occurred in 104 patients (46%) in the thrombectomy group and in 26 patients (23%) in the control group (adjusted rate ratio 2.06, 95% CI 1.46 to 2.91; P<0.001).
However, thrombectomy in these patients was associated with procedural complications and more symptomatic intracranial haemorrhages at 24-72 hours (12 patients [5%] with thrombectomy vs. none with control).
Following review of the evidence, the National Clinical Guideline for Stroke for the UK and Ireland advises caution when considering mechanical thrombectomy for patients presenting between 12 and 24 hours of onset and/or over the age of 80 because there are very limited data for these patients.[68]
Monitor the patient’s clinical status closely and provide supportive care as appropriate.[68] In particular, monitor:
Level of consciousness
Blood glucose
Blood pressure
Oxygen saturations
Hydration
Temperature
Cardiac rhythm and rate.
Monitor the patient for complications such as signs of raised intracranial pressure and seizures.
Level of consciousness
Assess and monitor the patient’s level of consciousness using the Glasgow Coma Scale. [ Glasgow Coma Scale Opens in new window ]
In patients with decreased level of consciousness or coma, urgently exclude haemorrhage and stroke mimics such as seizures. See Differentials.
Practical tip
Haemorrhagic stroke is more often associated with seizures, decreased level of consciousness, and signs of increased intracranial pressure than ischaemic stroke.
Blood glucose
Monitor blood glucose regularly. Maintain a blood glucose concentration between 4 and 11 mmol/L in people with acute stroke.[67]
Evidence: Glycaemic control in acute stroke
Despite evidence of an increased risk of poor functional outcome and death in patients with ischaemic stroke who develop post-stroke hyperglycaemia, limited available data do not show a significant benefit with tight glycaemic control compared with usual care. In addition, there is an increased risk of hypoglycaemia with tight glycaemic control.[67][68] [149][150][151]
A Cochrane systematic review found no significant difference in terms of dependency or death when intensive insulin therapy was compared with usual care to maintain blood glucose levels in adults with acute stroke, but rates of hypoglycaemia were higher with intensive insulin therapy.[152]
The review (search date September 2013) included 11 randomised controlled trials (RCTs), involving a total of 1583 participants (with and without diabetes) with blood glucose levels >6.1 mmol/L after a stroke.
The intensive insulin treatment intervention aimed to maintain blood glucose within the normal range of 4 to 7.5 mmol/L and was started in the first 24 hours of ischaemic stroke. Control interventions included placebo, no treatment, or loose control with insulin.
The mean blood glucose level during treatment was significantly lower in the intensive insulin intervention group than in the control group (6.7 mmol/L vs. 7.3 mmol/L; mean difference -0.63, 95% CI -0.80 to -0.46 mmol/L).
There was no significant difference between the treatment and control groups for the primary outcome of dependency or death (OR 0.99, 95% CI 0.79 to 1.23), or final neurological deficit (standardised mean difference -0.09, 95% CI: -0.19 to +0.01).
However, there was a significant increase in the incidence of symptomatic hypoglycaemia in the intensive treatment group compared with the control group (OR 14.6, 95% CI 6.6 to 32.2).
Results from an RCT including 1151 adults who received either intensive treatment for hyperglycaemia (target blood glucose concentration of 4.4 to 7.2 mmol/L) or standard treatment (target glucose concentration of 4.4 to 9.9 mmol/L) for up to 72 hours did not show a significant difference in favourable functional outcome at 90 days. Treatment was stopped early for hypoglycaemia or other adverse events in 11.2% of patients in the intensive treatment group and in 3.2% in the standard treatment group.[151]
Guidelines vary in their recommendations for target range for blood glucose in this situation.
The UK National Institute for Health and Care Excellence (NICE) guideline on stroke from 2008 recommends keeping blood glucose between 4 to 11 mmol/L (this was not changed in the 2022 update of this guideline).[67]
This recommendation is underpinned by:
Evidence from the United Kingdom Glucose Insulin in Stroke Trial (GIST-UK), which found no support for tight blood glucose control in patients with mildly or moderately elevated blood glucose levels following stroke[153]
Consensus of the NICE guideline panel on the recommended glucose range.
The guideline panel agreed by consensus that patients with pre-existing diabetes should continue to be treated according to current guidelines.
The 2016 National Clinical Guideline published by the Royal College of Physicians (updated in 2023 as the National Clinical Guideline for Stroke for the UK and Ireland) recommended a broader target range of 5 to 15 mmol/L, with close monitoring to avoid hypoglycaemia.[68]
This guideline cites the same trial as the NICE guideline to support this recommendation.[153]
The recommendation in the European Stroke Organisation guidelines from 2018 is underpinned by evidence from a systematic review including almost all the same studies as those included in the 2014 Cochrane review (above).[150]
This guideline makes a weak recommendation against the routine use of intravenous insulin to achieve tight glycaemic control as a means to improve functional outcome, survival, or infarct size (low-quality evidence as assessed using GRADE).[150]
Give optimal insulin therapy with intravenous insulin and glucose to all adults with type 1 diabetes with threatened or actual stroke. Follow your local protocol.[67]
Blood pressure
Monitor blood pressure regularly and give antihypertensive treatment only if there is a hypertensive emergency with one or more of the following serious concomitant conditions:[67]
Hypertensive encephalopathy
Hypertensive nephropathy
Hypertensive cardiac failure/myocardial infarction
Aortic dissection
Pre-eclampsia/eclampsia.
The National Institute for Health and Care Excellence (NICE) in the UK recommends to consider reducing blood pressure to 185/110 mmHg or lower in people who are candidates for intravenous thrombolysis.[67][117] In patients with acute ischaemic stroke not treated with intravenous thrombolysis or mechanical thrombectomy and blood pressure >220/120 mmHg, the European Stroke Organisation states that careful blood pressure reduction (<15% systolic blood pressure reduction in 24 hours) is reasonable and likely to be safe.[117] In patients on antihypertensive medication, resume oral treatment once the patient is medically stable and as soon as they can swallow medication safely.[68]
Evidence: Lowering blood pressure
The 2019 UK National Institute for Health and Care Excellence (NICE) guideline on stroke recommends that blood pressure should not be lowered routinely after an ischaemic stroke unless there are serious coexisting medical issues.[67]
The NICE guideline committee reported a lack of evidence from their analysis of two Cochrane reviews and four additional randomised controlled trials for benefit in manipulating blood pressure using beta-blockers or calcium-channel blockers in the first 72 hours of acute ischaemic stroke compared with control/placebo.[154][155] However, they agreed there may be individual clinical circumstances where active management of severe hypertension would be indicated.
A Cochrane systematic review (search date 2014) addressed the question of whether to continue or discontinue existing antihypertensive regimens in patients with acute stroke.[156]
The review included 26 trials involving a total of 17,011 adults with acute ischaemic stroke or intracerebral haemorrhage. Interventions for lowering blood pressure included alpha-blockers, ACE inhibitors, angiotensin-II receptor antagonists, calcium-channel blockers, nitric oxide donors, or thiazide-like diuretics.
Among the patients with ischaemic stroke who were not already on antihypertensive treatment, blood pressure lowering did not significantly change the following outcomes: “death or dependency” (OR 1, 95% CI 0.92 to 1.08; 8 trials; n=11,015), “death” (OR 0.95, 95% CI 0.78 to 1.16; 10 trials; n=11,238), “dependency” (mean difference in Barthel Index 0.84, 95% CI -3.21 to +4.89; 2 trials; n=3,681), “early neurological deterioration” (OR 0.58, 95% CI 0.09 to 3.82; 2 trials; n=3349), “length of stay” (mean difference -0.03; 95% CI -0.33 to +0.28; 2 trials; n=7393) or “quality of life” (mean difference in EuroQol 0.13, 95% CI -0.14 to +0.4; 2 trials; n=4038).
Among the patients with ischaemic stroke who were already taking antihypertensives who continued or stopped this treatment, there was no significant difference in the following outcomes: “death or dependency” at day 90 (OR 1.06, 95% CI 0.87 to 1.28; 1 trial; n=1,832), “death” (OR 1.24, 95% CI 0.96 to 1.61; 1 trial; n=1,832), “early neurological deterioration” (OR 1.27, 95% CI 0.89 to 1.82; 1 trial; n=2097), or “length of stay” (mean difference 1.2, 95% CI -0.87 to +3.27; 1 trial; n=2097). However, “dependency” was lower among those who stopped treatment (mean difference on the Barthel Index -3.8, 95% CI -7.23 to -0.37; 1 trial; n=2097) and “quality of life” was better among those who stopped treatment (mean difference on the EuroQol -0.03, 95% CI -0.06 to 0.00; 1 trial; n=2097).
Oxygen saturations
Monitor oxygen saturations and give supplemental oxygen only if oxygen saturation drops below 93%.[102] See Stabilisation under Urgent initial management above for more information.
Monitor controlled oxygen therapy. An upper SpO 2 limit of 96% is reasonable when administering supplemental oxygen to most patients with acute illness who are not at risk of hypercapnia. Evidence suggests that liberal use of supplemental oxygen (target SpO 2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[103] A lower target SpO 2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[104]
Do not routinely give oxygen to people who are not hypoxic.[67]
Hydration
Assess the patient’s hydration within 4 hours of their arrival at hospital.[67] Review regularly; manage as needed to maintain normal hydration.[67][68]
Temperature
Monitor temperature.[68][105] Patients with stroke can lose their thermoregulation acutely and may need interventions even in the absence of infection.
Give an antipyretic (e.g., paracetamol) in patients with high temperature. Do not give antipyretics to patients with a normal temperature to prevent hyperthermia.[105]
The National Institute for Health and Care Excellence (NICE) in the UK does not recommend the use of therapeutic hypothermia (a cooling device used to lower the body’s temperature by 2ºC to 4°C for several hours immediately after a stroke) to reduce the risk of secondary brain damage.[157]
Cardiac rhythm and rate
In people who would be eligible for secondary prevention treatment for atrial fibrillation or flutter:[68]
Perform prolonged ECG monitoring (at least 24 hours)
Consider prolonged sequential or continuous ECG monitoring with an external patch, wearable recorder, or implantable loop recorder in those in whom no other cause of stroke has been found, particularly if they have a pattern of cerebral ischaemia on brain imaging suggestive of cardioembolism.
Atrial fibrillation is an independent risk factor for ischaemic stroke and indicates a poor prognosis.[29] See New-onset atrial fibrillation.
Intracranial pressure
Monitor the patient for signs of elevated intracranial pressure (ICP). For information on other complications, see Complications.
Repeat the CT head immediately if you suspect elevated ICP, which may present as:
A reducing level of consciousness
Severe headache
Nausea/vomiting
A sudden increase in blood pressure.
Refer immediately to a neurosurgeon any patients with large middle cerebral artery territory infarcts and those with large infarctions affecting the cerebellum. These types of stroke have a very high mortality if urgent neurosurgical intervention is delayed.[69]
Patients with large middle cerebral artery territory infarcts (at risk of malignant middle cerebral artery syndrome) may need decompressive hemicraniectomy (neurosurgical removal of part of the skull to reduce intracerebral pressure).[69]
Patients with large infarctions affecting the cerebellum may need ventriculostomy (placement of an external ventricular drain) or posterior fossa craniectomy.[69]
Practical tip
Patients with large infarctions affecting the cerebellum or middle cerebral artery are at risk of developing oedema and elevated intracranial pressure. If left unchecked, the oedema compromises blood flow and causes brain herniation, which is frequently fatal.
In line with recommendations from the National Institute for Health and Care Excellence (NICE) in the UK, decompressive hemicraniectomy should be considered (performed within 48 hours of symptom onset) in any patient who meets all of the following criteria:[67]
Clinical deficits that suggest infarction in the territory of the middle cerebral artery, with a score >15 on the NIHSS [ NIH Stroke Score Opens in new window ]
Decreased level of consciousness, with a score of 1 or more on item 1a of the NIHSS
Signs on CT of an infarct of at least 50% of the middle cerebral artery territory:
With or without additional infarction in the territory of the anterior or posterior cerebral artery on the same side
or
With infarct volume greater than 145 cm 3, as shown on diffusion-weighted MRI scan.
Evidence: Decompressive hemicraniectomy
Decompressive hemicraniectomy reduces mortality, with some evidence of improved functional outcomes (although overall functional outcomes are poor in this population) and a variable impact on quality of life (which is generally low with or without surgery). There is no evidence that there should be an age cut-off for surgery, with the patient’s pre-stroke functional status being a more useful indicator of the potential outcomes of surgery.
There has been much debate about the net benefits of hemicraniectomy, especially in patients aged over 60 years due to the possibility of an increased risk of surviving with a serious disability, compared with people under 60 years.
The 2008 UK National Institute for Health and Care Excellence (NICE) guideline recommended hemicraniectomy only in people aged under 60 years. This was updated in 2019 to recommend that patients or their family members or carers should be given specific information on the risks and benefits in terms of functional outcomes and risk of mortality, so that personal values and preferences, especially regarding disability, are considered in shared decision-making.[158] This updated recommendation followed new evidence focusing on patient age, in particular the 2014 DESTINY II trial.[158]
The review by NICE found 5 randomised controlled trials (RCTs) of patients with an average age <60 years (HeMMi, HeADDFIRST, HAMLET, DESTINY and DECIMAL).[158]
In these studies, decompressive hemicraniectomy reduced mortality at 30 days (1 study; n=32; risk difference 416 fewer per 1000 [95% CI 64 fewer to 501 fewer]; moderate-quality evidence assessed using GRADE), 6 months (3 studies; n=86; risk difference 262 fewer per 1000 [95% CI 76 fewer to 371 fewer]; GRADE moderate) and 1 year (3 studies; n=134; risk difference 392 fewer per 1000 [95% CI 261 fewer to 469 fewer]; GRADE high).
There was a clinically important difference in functional outcomes (score 0 to 3 on modified Rankin Scale) at 6 months (risk difference 110 more per 1000 [95% CI 68 fewer to 441 more]; GRADE very low) or 1 year (risk difference 130 more per 1000 [95% CI 25 fewer to 392 more]; GRADE low).
There was no clinically important difference for quality of life at 1 year as measured by the SF-36 mental summary score (1 study; n=35; GRADE very low) or as measured using visual analog scales (1 study; n=32; GRADE low), but a clinically important harm of surgery for the SF-36 physical summary scale (1 study; n=35; GRADE low). Overall, quality of life scores were low in both groups.
NICE found 3 RCTs in patients with an average age over 60 years (including DESTINY II).[158]
In these studies, decompressive hemicraniectomy reduced mortality at 6 months (1 study; n=29; risk difference 487 fewer per 1000 [95% CI 122 fewer to 579 fewer]; GRADE moderate) and 1 year (3 studies; n=162; risk difference 364 fewer per 1000 [95% CI 227 fewer to 462 fewer]; GRADE moderate).
There was no difference in functional outcomes between groups at 6 months (2 studies; n=141; risk difference 23 more per 1000 [95% CI 7 fewer to 159 more]; GRADE very low) but a significant benefit for surgery at 12 months (3 studies; n=165; risk difference 100 more per 1000 [95% CI 10 more to 180 more]; GRADE very low).
Quality of life was higher at 1 year after surgery (1 study; n=100; GRADE low). Overall quality of life scores were low in both groups.
In all of the trials in people aged over 60 years, surgery had to be within 48 hours of symptom onset. Two of the trials in those aged under 60 years included people who had surgery longer than 48 hours after symptom onset (HeMMi 72 hours and HAMLET 96 hours). The NICE guideline group felt the beneficial results were largely driven by studies which only allowed surgery up to a maximum of 48 hours after onset, hence the time limit for surgery in their recommendation.
The NICE guideline committee notes that there was a clear mortality benefit of surgery at any age and that the patient’s pre-morbid state is more important than their age when making a decision about the risks of surgery. However, survivors have a high likelihood of moderate or severe disability with or without surgery.
Discuss the risks and benefits of the procedure with the patient or their family members or carers. Take into account the patient’s functional status before the stroke, and their wishes and preferences.[67] A shared decision-making process should include a careful discussion with the patient or their representatives about the risk of survival with substantial disability.[159]
Seizures
Consult immediately with a neurologist or neurosurgeon if the patient has uncontrolled or recurrent seizures, or status epilepticus. The choice of anticonvulsant will depend on individual patient characteristics.[69] See Status epilepticus.
Follow your hospital protocol. In practice, levetiracetam and sodium valproate are commonly used.
In people with non-disabling ischaemic stroke (i.e., minor stroke) who are not candidates for hyperacute treatment with thrombolysis and/or mechanical thrombectomy, secondary prevention of stroke assessment and management may be prioritised. See Prevention.
A minor ischaemic stroke is defined as a score <3 on the NIHSS, and no persistent disabling neurological deficit.[160] [ NIH Stroke Score Opens in new window ]
Antiplatelet therapy
Offer an antiplatelet agent as soon as possible but certainly within 24 hours (unless contraindicated) to any patient presenting with acute stroke who has had intracerebral haemorrhage excluded by imaging.[67] In practice, administration of aspirin (or alternative) is usually delayed 24 hours after alteplase, once a further CT scan has excluded significant bleeding.
The National Clinical Guideline for Stroke for the UK and Ireland recommends to offer:[68]
Aspirin (only) to patients with disabling ischaemic stroke. Continue aspirin daily until 2 weeks after the onset of stroke, then start definitive long-term antithrombotic treatment. Patients being transferred to care at home before 2 weeks should be started on long-term treatment earlier.[68]
Dual antiplatelet therapy with either aspirin and clopidogrel for 21 days, or aspirin and ticagrelor for 30 days in patients presenting within 24 hours of minor stroke and with a low risk of bleeding. For patients with minor ischaemic stroke who are not appropriate for dual antiplatelet therapy, give clopidogrel monotherapy.[68] After the completion of dual antiplatelet therapy, for long-term prevention of vascular events in people with ischaemic stroke without paroxysmal or permanent atrial fibrillation, single antiplatelet treatment should be used. See Secondary prevention.
However, the National Institute for Health and Care Excellence (NICE) in the UK recommends to offer:[67][161][162]
Aspirin orally (for those with no dysphagia), or
Aspirin rectally or by enteral tube (for those with dysphagia).
NICE recommends to offer an alternative antiplatelet agent to anyone who is allergic to or genuinely intolerant of aspirin.[67] In practice, clopidogrel is often used.
The European Stroke Organisation (ESO) recommends that aspirin and clopidogrel should be combined in adults with minor ischaemic stroke (NIHSS ≤3) within 24 hours of the stroke event. The ESO recommends that dual therapy should be continued for 21 days followed by single antiplatelet therapy.[163] In March 2021, the ESO guidelines made a weak recommendation based on moderate-quality evidence for 30 days of dual antiplatelet therapy with aspirin and ticagrelor in people with non-cardioembolic mild to moderate ischaemic stroke in the past 24 hours.[163] The ESO stated that this regimen should be considered as an alternative to aspirin plus clopidogrel, particularly in people known to be intolerant of clopidogrel or in people who have moderate stroke (NIHSS 4 or 5) and no other contraindication.[163] The use of dual antiplatelet therapy with aspirin and ticagrelor is also supported by the European Society of Cardiology.[164] However, an application to the European Medicines Agency (EMA) to change the marketing authorisation of ticagrelor to include the prevention of stroke in adults who have had a mild to moderate ischaemic stroke was withdrawn in December 2021. Based on trial data and the company’s response to their questions, the EMA expressed concern that the benefits of short-term treatment with ticagrelor plus aspirin in preventing stroke in these patients did not clearly outweigh the risks of fatal and non-fatal bleeding.
Antiplatelet treatment may be contraindicated or delayed in patients with active bleeding (e.g., from the gastrointestinal tract).
Evidence: Dual antiplatelet therapy with aspirin and clopidogrel
Guidelines from the National Clinical Guideline for Stroke for the UK and Ireland and from the European Stroke Organisation recommend to consider dual antiplatelet therapy (DAPT) with aspirin and clopidogrel in patients presenting within 24 hours of minor stroke (e.g., NIHSS 0-3) and with a low risk of bleeding, followed by single antiplatelet therapy, if there are no contraindications.[68][163]See panel below for evidence supporting DAPT with aspirin and ticagrelor.
The National Clinical Guideline for Stroke for the UK and Ireland cites the CHANCE and POINT trials to support the use of DAPT with aspirin and clopidogrel.[165][166][167] Guidance from the European Stroke Organisation additionally cites the FASTER trial.[163][168]
CHANCE[165]
This trial was conducted at 114 centres in China and recruited 5170 patients.
In patients with high-risk transient ischaemic attack (TIA) or minor ischaemic stroke (NIHSS 0-3), DAPT with clopidogrel plus aspirin started within 24 hours of onset for 21 days resulted in a significant reduction in ischaemic stroke over the first 90 days from 11.7% with aspirin plus placebo versus 8.2% with aspirin plus clopidogrel (hazard ratio [HR] 0.68, 95% CI 0.57 to 0.81; P<0.001).
DAPT with clopidogrel plus aspirin did not increase the risk of moderate or severe haemorrhage in comparison with aspirin alone. The benefit persisted during 1-year follow-up.[169]
POINT[166]
This trial recruited 4881 patients with high-risk TIA or minor ischaemic stroke (NIHSS 0-3) across 269 international sites in North America, Europe, Australia, and New Zealand.
DAPT with clopidogrel plus aspirin, started within 12 hours of onset and continued for 90 days, resulted in a significant reduction of ischaemic stroke from 6.5% with aspirin alone versus 5.0% with aspirin plus clopidogrel (HR 0.75, 95% CI 0.59 to 0.95; P=0.02), with most events occurring during the first week after the initial event.
The trial was stopped early because of a lower risk of major ischaemic events and a higher increase in major haemorrhage (mostly gastrointestinal) in the DAPT group, with an absolute risk increase of 0.5%. There was no difference in fatal haemorrhage or intracranial haemorrhage.
The higher rate of bleeding in the POINT trial compared with the CHANCE trial was postulated to be due to a shorter duration of combined treatment in the CHANCE trial (21 days in the CHANCE trial vs. 90 days in the POINT trial) and differences in the metabolism of clopidogrel in Asian versus non-Asian people.[170]
The National Clinical Guideline for Stroke for the UK and Ireland and the European Stroke Organisation cite a pooled analysis of these two trials, finding benefit of DAPT with aspirin plus clopidogrel in this population was confined to the first 21 days.[171]
FASTER[168]
This trial, conducted in patients in North America, compared DAPT with aspirin and clopidogrel with aspirin alone within 24 hours of symptom onset in patients with TIA or minor ischaemic stroke.
At 90 days, it found a 7.1% stroke rate on combined therapy versus 10.8% on aspirin alone (risk ratio [RR] 0.7, 95% CI 0.3 to 1.2; absolute risk reduction -3.8%, 95% CI -9.4 to 1.9; P=0.19).
There was a statistically non-significant increase in symptomatic bleeding with aspirin and clopidogrel compared with aspirin alone.
A 2018 systematic review of these three randomised placebo-controlled trials (CHANCE, FASTER, POINT; n=10,447), found:[172]
Compared with aspirin alone, DAPT (clopidogrel and aspirin) started within 24 hours of high-risk TIA or minor ischaemic stroke reduced the risk of non-fatal recurrent stroke by about 20 in 1000 population at 90 days (RR 0.70, 95% CI 0.61 to 0.81; absolute reduction 1.9%).
There was a possible increase in moderate to severe extracranial bleeding of 2 per 1000 population.
The reviewers concluded that discontinuation of dual antiplatelet therapy within 21 days and possibly as early as 10 days of initiation was likely to maximise benefit and minimise harm.
Evidence: Dual antiplatelet therapy with aspirin and ticagrelor
In its guidance on DAPT, the National Clinical Guideline for Stroke for the UK and Ireland also recommends aspirin and ticagrelor if there are no contraindications (as an equal alternative to dual treatment with aspirin and clopidogrel) for 30 days in patients presenting within 24 hours of minor stroke (e.g., NIHSS 0-3) and with a low risk of bleeding.[68]
This guidance is supported by the THALES randomised controlled trial of 11,016 patients with high-risk transient ischaemic attack or minor ischaemic stroke (NIHSS ≤5), none of whom received thrombolysis or thrombectomy or required anticoagulation.[167]
THALES demonstrated that compared with aspirin plus placebo, dual treatment with ticagrelor and aspirin reduced the risk of disabling stroke or death within 30 days.
The primary composite outcome of recurrent stroke or death occurred in 303 patients (5.5%) in the dual antiplatelet group and in 362 patients (6.6%) in the aspirin plus placebo group (hazard ratio [HR] 0.83, 95% CI 0.71 to 0.96; P=0.02).
Ischaemic stroke occurred in 276 patients (5.0%) in the ticagrelor plus aspirin group and in 345 patients (6.3%) in the aspirin plus placebo group (HR 0.79, 95% CI 0.68 to 0.93; P=0.004).
Severe bleeding was more frequent with ticagrelor plus aspirin (0.5%) than with aspirin plus placebo (0.1%) (HR 3.00, 95% CI 1.74 to 9.14; P=0.001), including intracranial haemorrhage (0.4% with ticagrelor plus aspirin vs. 0.1% aspirin plus placebo) (HR 3.33, 95% CI 1.34 to 8.28; P=0.01).
Offer a proton-pump inhibitor, in addition to aspirin, to any patient who reports previous dyspepsia associated with aspirin.[67] A proton-pump inhibitor should be considered for concurrent use with dual antiplatelet therapy to reduce the risk of gastrointestinal haemorrhage.[68]
Practical tip
Many ischaemic strokes are caused by conventional atherosclerosis, either in major vessels (such as the aortic arch or carotid arteries) or more distal vessels in the brain itself. Strokes caused by atherosclerosis, plaque rupture, platelet aggregation, and vessel occlusion by platelet-rich thrombus or embolism of the thrombus should be treated with antiplatelet agents.[67][68]
Anticoagulation
Do not use anticoagulant treatment routinely to treat acute stroke.[67]
[  ]
[Evidence A]
]
[Evidence A]
Evidence: Anticoagulation
There is evidence from one systematic review that early anticoagulation shows no net benefit in acute ischaemic stroke.
A Cochrane systematic review (search date June 2014) assessed the effectiveness and safety of early (within the first 14 days of onset; >90% within 48 hours) anticoagulation (with unfractionated heparin, low molecular weight heparin, heparinoids, direct oral anticoagulants, and thrombin inhibitors) in people with acute presumed or confirmed ischaemic stroke. The review included 24 randomised controlled trials involving a total of 23,748 participants.[173]
At the end of follow-up, anticoagulants did not reduce the risk of death (OR 1.05, 95% CI 0.98 to 1.12; 11 trials; 22,776 participants), or death or dependency (OR 0.99, 95% CI 0.93 to 1.04; 8 trials; 22,125 participants).
Anticoagulants significantly reduced the odds of deep vein thrombosis (OR 0.21, 95% CI 0.15 to 0.29; 10 trials; 916 participants), although most deep vein thromboses detected were subclinical and asymptomatic.
Anticoagulants also significantly reduced the odds of fatal or non-fatal symptomatic pulmonary embolism (OR 0.60, 95% CI 0.44 to 0.81; 14 trials; 2,544 participants) and recurrent ischaemic stroke (OR 0.76, 95% CI 0.65 to 0.88; 11 trials; 21,605 participants).
However, anticoagulation significantly increased symptomatic (fatal or non-fatal) intracranial haemorrhages (OR 2.55, 95% CI 1.95 to 3.33; 16 trials, 22,943 participants) and major extracranial haemorrhage (defined as bleeding serious enough to cause death or require hospitalisation or transfusion; OR 2.99, 95% CI 2.24 to 3.99; 18 trials; 22,255 participants).
The authors of the review concluded that the data do not support the routine use of early anticoagulants in acute ischaemic stroke.
The 2008 UK guideline on stroke from the National Institute for Health and Care Excellence (NICE) made recommendations on the use of anticoagulation in patients with stroke based on evidence from an earlier version of this Cochrane review (search date 2003).[67][174]
These recommendations are unchanged in the most recent version (2022 update) of this NICE guideline.[67]
The National Clinical Guideline for Stroke for the UK and Ireland highlights other considerations on the use of anticoagulation: for example, in people with concomitant atrial fibrillation or flutter. See below.
Indications for anticoagulation in people with stroke include:[67]
A cardiac source of embolism
Cerebral venous thrombosis
Some cases of arterial dissection.
Cerebral microbleeds (regardless of number or distribution) need not preclude the use of anticoagulation.[68] Anticoagulation should include measures to reduce bleeding risk, using a validated tool to identify modifiable risk factors.[68]
See Complex presentations, below, for more information on anticoagulation treatment in complex presentations.
Practical tip
The patient receiving thrombolysis on presentation should not necessarily affect when you start anticoagulation. If there has been significant haemorrhagic transformation of the infarction some clinicians would delay starting anticoagulation beyond 2 weeks, but there are no clear guidelines covering this area of practice. If there is a full recovery with thrombolysis (or mechanical thrombectomy), anticoagulation could and should be considered significantly earlier.[67]
In terms of venous thromboembolism (VTE) prophylaxis in patients with ischaemic stroke, there may be some people for whom the risk of VTE outweighs the risk of haemorrhagic transformation. People at particularly high risk of VTE include anyone with:[67]
Complete paralysis of the leg
A previous history of venous thromboembolism
Dehydration
Comorbidities (such as malignant disease)
Current or recent smoker.
Regularly review the patient if they are given prophylactic anticoagulation.[67]
Practical tip
Haemorrhagic conversion is most common in larger infarcts and in those receiving anticoagulation or alteplase. See Complications.
Complex presentations
Seek senior advice for the management of more complex presentations, such as:
Acute venous stroke
Stroke associated with arterial dissection
Either anticoagulants or antiplatelet agents are indicated in patients who have a stroke secondary to acute arterial dissection.[67]
Stroke associated with antiphospholipid syndrome
Manage in the same way as acute ischaemic stroke without antiphospholipid syndrome. There is insufficient evidence to support any recommendations on safety and efficacy of anticoagulants versus antiplatelet agents in this sub-population.[67]
Atrial fibrillation or atrial flutter
Anticoagulation should be considered in people with stroke and paroxysmal, persistent, or permanent atrial fibrillation or atrial flutter once intracranial bleeding and other contraindications (such as severe uncontrolled hypertension - clinic blood pressure of 180/120 or higher, which should be treated first) are excluded.[68]
Disabling ischaemic stroke
The National Clinical Guideline for Stroke for the UK and Ireland recommends that patients with moderate to severe ischaemic stroke and atrial fibrillation or flutter may be considered for anticoagulation from 5-14 days of onset for moderate to severe stroke. Aspirin should be used in the meantime.[68] Wherever possible, these patients should be offered participation in a trial of the timing of initiation of anticoagulation after stroke.[68]
However, NICE recommends to defer anticoagulation until at least 2 weeks from onset of symptoms.[67]
Non-disabling ischaemic stroke
The National Clinical Guideline for Stroke for the UK and Ireland recommends that patients with ischaemic stroke and atrial fibrillation or flutter should be considered for anticoagulation within 5 days of onset for mild stroke.[68] Aspirin should be used in the meantime.[68]
NICE recommends to defer anticoagulation for an interval at the discretion of the prescriber, but no later than 2 weeks from the onset of symptoms.[67]
First-line treatment for people with ischaemic stroke due to non-valvular atrial fibrillation should be anticoagulation with a direct oral anticoagulant (DOAC).[68]
People with ischaemic stroke due to valvular/rheumatic atrial fibrillation or with mechanical heart valve replacement, and those with contraindications or intolerance to DOAC treatment, should receive anticoagulation with adjusted-dose warfarin (target INR 2.5, range 2.0 to 3.0) with a target time in the therapeutic range of greater than 72%.[68]
For people with cardioembolic transient ischaemic attack or stroke for whom treatment with anticoagulation is considered inappropriate because of a high risk of bleeding:[68]
Antiplatelet treatment should not be used as an alternative when there are absolute contraindications to anticoagulation (e.g., undiagnosed bleeding)
A left atrial appendage occlusion device may be considered as an alternative, provided the short-term peri-procedural use of antiplatelet therapy is an acceptable risk.
For people with cardioembolic transient ischaemic attack or stroke for whom treatment with anticoagulation is considered inappropriate for reasons other than the risk of bleeding:[68]
Antiplatelet treatment may be considered to reduce the risk of recurrent vaso-occlusive disease.
Atrial fibrillation causes at least one fifth of ischaemic strokes and is one of the strongest individual stroke risk factors.[80]
Patients with prosthetic valves and at risk of haemorrhage
Stop anticoagulation treatment for 1 week and substitute aspirin.[67]
Symptomatic proximal deep vein thrombosis or pulmonary embolism
Treat with anticoagulation in preference to aspirin unless there are other contraindications to anticoagulation.[67]
Statins
Do not start statin treatment immediately. Usually it would be appropriate to start statin treatment once a patient can swallow medication safely, and there is consensus that it is safe to start statins after 48 hours.[67][68]
Offer high-intensity statin therapy (unless contraindicated or investigation confirms no evidence of atherosclerosis) to all patients.[68]
A lower dose should be used if there is the potential for medication interactions or a high risk of adverse effects.
Use an alternative statin at the maximum tolerated dose if a high-intensity statin is unsuitable or not tolerated.
Continue statin treatment in people who are already receiving statins.[67] Consider increasing the statin intensity or dose if the patient is not currently taking a high-intensity statin at the maximum tolerated dose.[61]
People with ischaemic stroke in whom investigation confirms no evidence of atherosclerosis should be assessed for lipid-lowering therapy on the basis of their overall cardiovascular risk.[68]
Swallowing assessment and nutrition
On admission, ensure the patient has their swallowing function assessed by appropriately trained staff before being given any oral food, fluid, or medication:[67][68]
If the admission screen indicates problems with swallowing, ensure specialist assessment within 24 hours of admission (preferably) and not more than 72 hours afterwards.
To avoid aspiration pneumonia, give food, fluids, and medication to people with dysphagia in a form that can be swallowed without aspiration, after specialist assessment of swallowing.[67]
Start nutrition support for people who are at risk of malnutrition. Routine nutritional supplementation is not recommended for people who are adequately nourished on admission.[67]
Optimal positioning and early mobilisation
Ensure patients have an initial specialist assessment for positioning as soon as possible and within 4 hours of arrival at hospital.[68] Assess individual clinical needs and personal preferences to determine the patient’s optimal head position. Take into account factors such as comfort, physical and cognitive abilities, and postural control.[67] Stroke units should not have a policy or practice that favours either a sitting up or lying-flat head position.[68] When lying or sitting, patients with acute stroke should be positioned to minimise the risk of aspiration and other respiratory complications, shoulder pain and subluxation, contractures, and skin pressure ulceration.[68]
Evidence: Optimal positioning
Evidence shows no difference in outcomes when the patient with acute ischaemic stroke is positioned lying flat or with their head elevated. Therefore the optimum position should be individually tailored to suit the patient.[176]
There is wide variation in clinical practice, with lying flat thought to help maintain blood flow to “at-risk” brain regions, but possibly increasing the risk of complications such as pneumonia. In 2019, the UK National Institute for Health and Care Excellence (NICE) performed a new systematic review for their stroke guideline comparing positioning patients with acute ischaemic stroke lying flat versus sitting up. The review included two studies, reported in six papers, both of PROBE (prospective, randomised, open-label, controlled trial with blinded outcome evaluation) design.[176]
In the pilot HeadPoST study (94 people), the intervention group lay flat for 24 hours, then from 24 to 48 hours had their heads raised slowly to a maximum of 15°; after 48 hours, heads were elevated further to the standard 30° or more. The control group sat up with heads elevated to 30° or more as soon as possible after the diagnosis, and maintained in this position for at least 48 hours.[177]
In the full HeadPoST study (11,093 people), the intervention group lay flat as soon as possible after presentation and for at least 24 hours. The control group sat up with heads elevated to at least 30° immediately upon presentation to the emergency department and for at least 24 hours.[178]
The NICE guideline review reported that:
Despite the pilot trial finding that there was a possible benefit with lying down in terms of function at 90 days, the larger full study did not find any difference between groups (modified Rankin Scale 0 to 2; 2 studies; n=9840; RR 1.07, 95% CI 0.9 to 1.26; GRADE very low quality).
There was no clinically important difference in recurrent stroke (2 studies; n=11,185; moderate quality evidence assessed using GRADE), pneumonia at 90 days (1 study; n=11,093; GRADE low quality), EQ-5D for pain/discomfort at 90 days (1 study; n=8830; GRADE low quality), length of stay (1 study; n=94; GRADE low quality) or mortality at 90 days (2 studies; n=10,945; GRADE moderate quality).
The guideline committee noted that large numbers of patients were excluded from enrolment due to clinician discretion (particularly in relation to their ability to tolerate the lying flat position) and that the average stroke severity was lower and not representative of the range of stroke severities managed within UK stroke centres.
They therefore concluded that individual factors such as comfort, medical condition, pressure care, pain, physical and cognitive abilities, orientation, alignment, postural control and compliance should be considered when positioning patients with acute stroke.[176]
Arrange assessment of patients with mobilisation difficulties by an appropriately trained healthcare professional as soon as possible. Ensure this assessment is conducted within the first 24 hours of onset to determine the most appropriate and safe methods of transfer and mobilisation.[68] Help the patient to sit out of bed, stand, or walk as soon as their clinical condition permits as part of an active management programme in a specialist stroke unit.[67] Mobilisation typically begins between 24 and 48 hours of stroke onset.[68]
[  ]
]
If the patient needs help to sit out of bed, stand, or walk, do not provide high-intensity mobilisation in the first 24 hours after symptom onset.[67][68]
High-intensity mobilisation refers to the very early mobilisation intervention from the AVERT trial.[68][179] It includes mobilisation that: begins within the first 24 hours of stroke onset; includes at least three additional out-of-bed sessions compared with usual care; focuses on sitting, standing, and walking (i.e., out of bed) activity.
Evidence: Early mobilisation (within 48 hours)
Guidelines suggest that early mobilisation (within 48 hours) may be appropriate in patients who require minimal assistance to mobilise (e.g., those who have had a mild stroke, or are experiencing language and/or upper limb dysfunction alone), although evidence is limited.[180]
In 2019, the UK National Institute for Health and Care Excellence (NICE) found two studies examining early (within 48 hours) mobilisation involving a total of 75 people.[180][181][182]
The review by NICE incorporating these two studies reported that there was no significant difference for the outcomes of mortality, functional outcome (modified Rankin Scale), neurological deterioration, adverse events, or length of hospital stay (all very low-quality evidence assessed using GRADE).
The guideline panel made a consensus recommendation that mobilisation should be considered as and when the patient’s clinical condition permits.
Evidence: Very early mobilisation (within 24 hours)
Very early mobilisation does not seem to improve outcomes, and high-intensity strategies may cause clinical harm in early functional outcomes, possibly due to reduced cerebral perfusion. Therefore very early mobilisation should not be actively pursued. However, patients who can mobilise with little or no help in the first 24 hours after stroke should not be discouraged from doing so.[68][180]
In 2019, the UK National Institute for Health and Care Excellence (NICE) performed a review for their stroke guideline and found six studies examining very early (within 24 hours) mobilisation involving a total of 2475 people.[180]
The majority of data was from the AVERT III 2016 trial.[179][183][184][185][186] This study used a high-intensity mobilisation strategy beginning within 24 hours of stroke symptom onset and including at least three additional out-of-bed (sitting, standing or walking) sessions compared with usual care.
For functional outcomes there was a suggestion of clinical harm as a result of very early mobilisation with fewer patients reaching a modified Rankin Scale 0 to 2 at 7 days (2 studies; n=191; 118 fewer per 1000 [from 223 fewer to 20 more], low-quality evidence assessed using GRADE); however there was no significant difference between very early mobilisation and usual care for mRS 0 to 2 at 90 days (5 studies; n=2377; high-quality evidence assessed using GRADE) or 12 months (2 studies; n=2152; moderate quality evidence assessed using GRADE)
There was also no significant difference between groups for recurrent stroke (1 study; n=71; low quality evidence assessed using GRADE), neurological deterioration (1 study; n=138; GRADE very low quality), adverse events (2 studies; n=209; GRADE moderate quality), length of hospital stay (1 study; n=124; GRADE low quality) or mortality at 90 days (6 studies; n=2475; GRADE moderate quality).
One small study found a statistically significant benefit of very early mobilisation for functional outcomes measured by the Barthel index at discharge (n=90; GRADE moderate quality) and at 90 days (n=80; GRADE moderate quality), but the guideline committee did not consider this clinically meaningful.[180]
NICE comments that evidence on very early mobilisation is difficult to interpret due to the differences in intensity, timing, and type of mobilisation used in the trials, and lack of stratification by initial ability to mobilise independently.[180]
The NICE guideline committee consensus is not to restrict appropriate very early (within 24 hours) mobilisation in people who are independently mobile after having a stroke. They advise not to start intense mobilisation (more frequent mobilisations of a longer duration than ‘usual care’) within the first 24 hours among people who need help to sit out of bed, stand, or walk, because this could reduce cerebral perfusion in these patients.
The National Clinical Guideline for Stroke for the UK and Ireland makes a similar recommendation that mobilisation within 24 hours of onset of stroke should only be for patients who require little or no assistance to mobilise, basing this decision on the results of the AVERT trial.[68][183]
Prevention of deep venous thrombosis and pulmonary embolism in immobile patients
Give intermittent pneumatic compression within 3 days of admission for the prevention of deep vein thrombosis and pulmonary embolism. Give continuous treatment for 30 days or until the patient is mobile or discharged, whichever is sooner.[68]
Do not routinely give low molecular weight heparin or graduated compression stockings.[68] However in practice, prophylactic low molecular weight heparin may be considered if intermittent pneumatic compression is contraindicated or not possible.
Offer information on stroke to patients and their family/carers.[68] See Patient leaflets.
Arrange follow-up in primary care on discharge.
How to insert a tracheal tube in an adult using a laryngoscope.
How to use bag-valve-mask apparatus to deliver ventilatory support to adults. Video demonstrates the two-person technique.
Use of this content is subject to our disclaimer