Approach
Paraesthesias can be caused by a wide range of conditions affecting the nervous system at any level. The clinical history and physical examination narrow the differential diagnosis and guide the need for further investigations. The aim of the examination is to determine whether the pathology is likely to be affecting the peripheral nerves, plexuses, dorsal spinal roots, spinal cord, or brain, and to identify additional signs of the underlying cause.
Diabetic neuropathy, hypocalcaemia, vitamin deficiencies, drug toxicity, and minor infections such as shingles or herpes simplex virus (HSV) can usually be diagnosed clinically or with laboratory testing. All other peripheral neuropathies may require electromyography (EMG) with nerve conduction studies to confirm and characterise the neuropathy. Imaging is required if the history and examination suggest a plexopathy, a radiculopathy, or a lesion affecting the spinal cord, brainstem, or brain.[Figure caption and citation for the preceding image starts]: Ascending sensory pathways of the spinal cord. The dorsal column system and spinothalamic tract are the major ascending pathways that connect the periphery with the brain.Betts JG, Young KA, Wise JA et al.Anatomy and physiology. Houston, TX:OpenStax; 2013 (CC BY4.0 - https://creativecommons.org/licenses/by/4.0/) [Citation ends].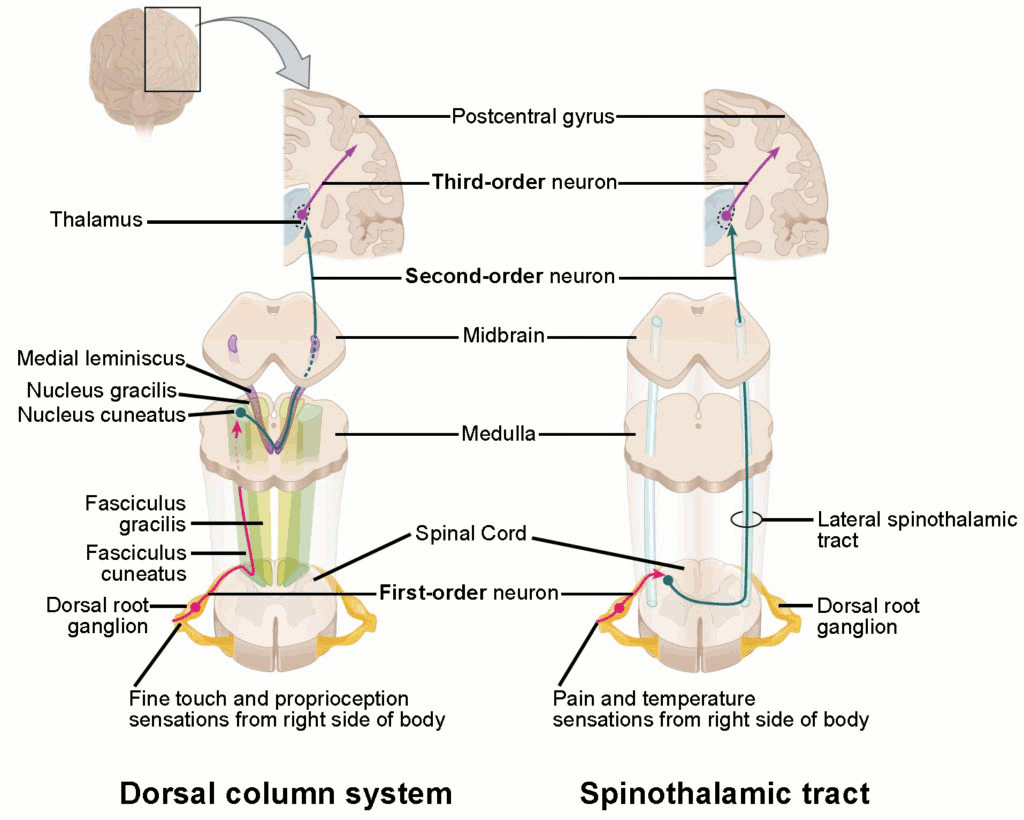
Characteristics of the paraesthesias
Description
It is important to characterise the paraesthesias being experienced by the patient. The patient should be encouraged to describe their symptoms in detail in their own words. Common descriptions include burning, stabbing, pins and needles, prickling, stinging, and sharp shooting pains. It is important to establish if there is an associated loss of sensation, and if it is in the same area as the paraesthesias. Painful paraesthesias suggest an inflammatory or ischaemic process such as vasculitis. Shooting pains are characteristic of nerve entrapment. Burning pains are characteristic of paraesthesias affecting small unmyelinated fibres. Paraesthesias may occur as part of a migraine aura or have an onset at the same time as the headache, and they typically last <1 hour from the onset of the headache.
Onset
It is important to establish if the symptoms had a sudden onset or if they evolved over seconds, minutes, hours, days, or weeks. A sudden onset suggests stroke or trauma. Symptoms that evolve over several seconds suggest epilepsy. Symptoms that evolve over minutes suggest migraine, panic attack, or fish poisoning (if the patient has ingested fish within the previous 8 hours). An insidious onset is characteristic of inherited neuropathies.
Duration and severity
The patient should be asked whether the symptoms are constant or relapsing and remitting, and whether there has been any symptom progression. A history of similar previous symptoms should be sought. Muscle pain, atrophy, or weakness in the same anatomical distribution as the paraesthesias indicates a sensori-motor peripheral neuropathy (often a sign of more advanced disease).
Localisation
The location of the symptoms indicates the level of the lesion.
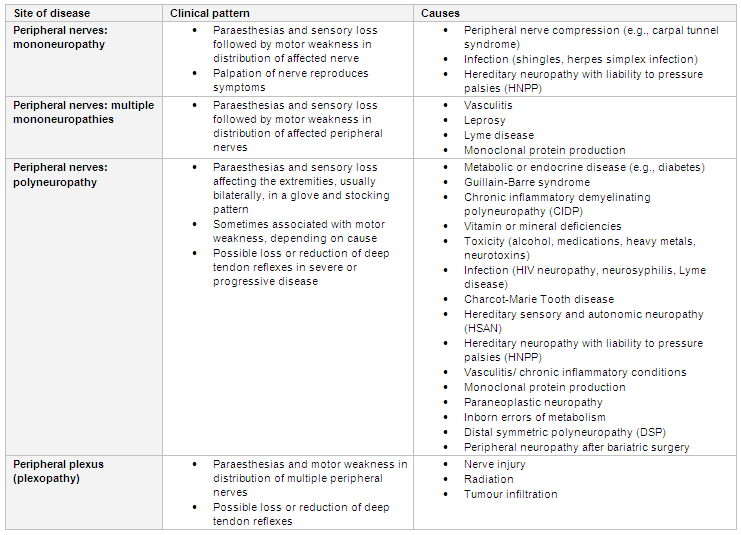
[Figure caption and citation for the preceding image starts]: Peripheral nervous system causes of paraesthesias according to the pattern and level of the lesionCreated by BMJ Knowledge Centre using information from Dr Caroline M. Klein [Citation ends].
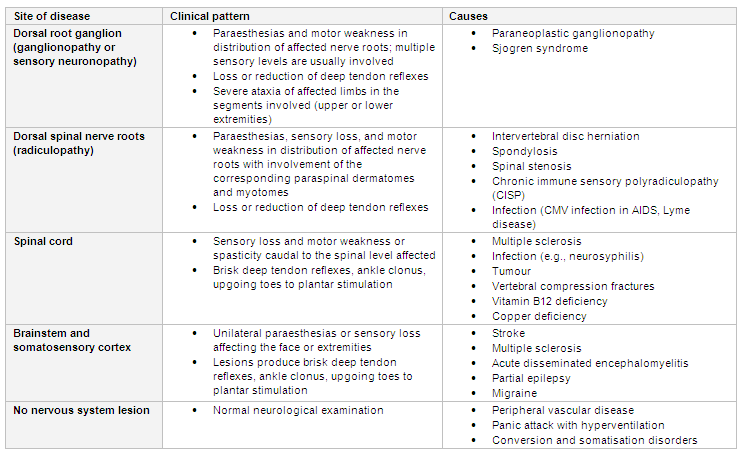
[Figure caption and citation for the preceding image starts]: Central nervous system and non-neurological causes of paraesthesias according to the pattern and level of the lesionCreated by BMJ Knowledge Centre using information from Dr Caroline M. Klein [Citation ends].
Localised symptoms indicate a peripheral mononeuropathy or a plexopathy if in the distribution of one or more peripheral nerves, or a radiculopathy if in the distribution of a dermatome. If symptoms suggest a peripheral mononeuropathy, a more detailed history should be taken to assess for focal nerve entrapment syndromes.
Paraesthesias affecting the first three digits of the affected hand suggest carpal tunnel syndrome. Symptoms are usually worse at night (awakening patient from sleep) and exacerbated by prolonged wrist extension such as driving, typing on a keyboard, or reading a newspaper. Patients may also have pain in the wrist or hand possibly extending into the forearm, elbow, or shoulder.
Paraesthesias affecting the fourth and fifth digits suggest ulnar neuropathy, which may be induced by prolonged or repetitive flexion of the elbow or repetitive leaning on the elbow.
Paraesthesias in the lateral leg or dorsal foot may indicate fibular (peroneal) neuropathy. Patients may also have a foot drop. The nerve compression is usually due to repetitive crossing of the knees or prolonged kneeling, crouching, or squatting, but a history of trauma or previous knee surgery may also be present.
Paraesthesias in the medial aspect of the foot suggest tibial neuropathy, which is relatively rare.
Burning paraesthesias with increased sensitivity to touch or pressure in the anterolateral thigh region suggest meralgia paraesthetica, produced by compression of the lateral cutaneous nerve of the thigh.
Paraesthesia characterised by persistent itching, localised unilaterally on the upper back, is called notalgia paraesthetica. Symptoms may include pain, tingling, numbness, or increased sensitivity to light touch in the affected area. Notalgia paraesthetica is thought to be due to compression of the dorsal or sensory branches of the spinal nerves, from dermatomes T2 to T6, by paraspinal muscle spasm or by bony degenerative changes in the spine at these levels.[64]
Paraesthesias in the distribution of the trigeminal nerve indicate trigeminal neuropathy.
Generalised unilateral symptoms involving the face or extremities suggest spinal cord or brain pathology. Muscle pain, atrophy, or weakness in the same anatomical distribution as the paraesthesias may also suggest involvement of a spinal nerve root, spinal cord, or brain. Spinal pain with radiation into the extremity where the paraesthesias are reported, or bowel or bladder incontinence, may indicate a radicular or spinal nerve root aetiology.
Unilateral paraesthesias or anaesthesia of the chin (numb chin syndrome) may present as an initial manifestation of occult malignancy and should always be investigated thoroughly as to the underlying cause.[12][54] It may also be the initial manifestation of multiple sclerosis. It may also occur subsequent to dental or oral surgery.
Specific patterns
Important patterns to recognise include rapidly progressive paraesthesias, numbness and weakness beginning in the extremities indicating acute inflammatory demyelinating polyradiculoneuropathy (AIDP), pain followed by paraesthesias, and weakness in multiple single peripheral nerve distributions indicating mononeuritis multiplex, or unilateral symptoms that may indicate brain or spinal cord lesions.
AIDP should be distinguished from chronic inflammatory demyelinating polyneuropathy (CIDP) and chronic inflammatory demyelinating sensory polyradiculopathy (CISP). CIDP can produce a similar progressive pattern as AIDP, but over a longer time course (8 weeks). CIDP can also cause relapsing and remitting symptoms. CISP produces slowly progressive sensory symptoms without motor symptoms.
Dying-back neuropathy is a pattern seen with various toxicities (including alcohol and hexane) in which symptoms begin in the lower extremities and gradually progress proximally, becoming painful and involving the upper extremities once symptoms reach ankle level.
Stereotyped episodes of hemifacial or hemibody sensory symptoms such as transient paraesthesias, and occurring in isolation or in association with automatisms or altered level of consciousness or responsiveness, should prompt suspicion of focal seizures. The paraesthesias typically evolve over a few seconds.
In distal symmetric polyneuropathy (DSP) there are symmetrically distributed symptoms of paraesthesias (usually tingling paraesthesias with or without associated asleep- or dead-type numbness in the same areas) and usually later muscle weakness. Symptoms are progressive from onset, and may be intermittently present initially, then become more constant. Symptoms begin in the toes and extend proximally in both legs to the level of the knees, at which point the distal fingertips of both hands typically become involved. If small sensory nerve fibres are affected, burning pains and allodynia (painful sensations elicited by normally non-noxious stimuli, such as light touch) are usually reported by the patient.
In peripheral neuropathy after bariatric surgery there are symmetric sensory symptoms of asleep- or dead-type numbness and paraesthesias in the hands and feet, occasionally with some muscle weakness in these same regions; insidious more often than acute or sub-acute onset of symptoms; aching, sharp, or burning pains in hands and feet.
General medical history
Constitutional symptoms
Weight loss, night sweats, and/or fatigue may be present in infection, neoplastic disease, or a range of inflammatory conditions. Dry eyes or mouth may indicate Sjogren's syndrome.
Skin and joint changes
Ulcers, purpura, rash, or darkening of the skin may suggest peripheral vascular disease, infection, vasculitis, monoclonal protein production, sarcoidosis, or an inborn error of metabolism. Arthralgias, joint swelling, or stiffness may indicate a rheumatological condition.
Cardiovascular/respiratory symptoms
Lightheadedness or syncope with standing may indicate autonomic dysfunction, which occurs in hereditary sensory and autonomic neuropathy, severe diabetic neuropathy, Sjogren's syndrome, and some inborn errors of metabolism. Chest pain or palpitations at the onset of the paraesthesias may indicate stroke. Chronic cough or shortness of breath may indicate diaphragmatic involvement due to pathology affecting the cervical spinal roots. An upper respiratory or gastrointestinal illness may precede the onset of AIDP.
Past medical illnesses
It is important to establish if the patient has any of the following underlying conditions: diabetes mellitus (level of control and complications of diabetic retinopathy and nephropathy), rheumatological disorders (systemic lupus erythematosus, Sjogren's syndrome, rheumatoid arthritis, vasculitides), cancer, spinal cord injury or surgery, infectious disease (HIV, hepatitis, syphilis), stroke, coronary artery disease, alcohol abuse, nutritional deficiency, renal failure (including symptoms of uraemia), or thyroid disease.
Surgical history
A history of bariatric surgery within the prior 12-24 month period, especially in association with rapid loss of a large amount of body weight, could be the cause of peripheral neuropathy after bariatric surgery.
Medication history
Current and previous therapies should be carefully documented. Known causative medications include chemotherapy agents (cisplatin, vincristine, cytosine arabinoside, thalidomide, paclitaxel), antibiotics (metronidazole, nitrofurantoin), antiretroviral agents (zidovudine, stavudine, lamivudine), and antiepileptics (phenytoin). Non-prescription vitamins and supplements should also be documented, especially those containing vitamin B6 (vitamin B6 overdose can cause a peripheral neuropathy). Previous radiotherapy to the axilla or pelvis may indicate radiation nerve damage, even if the exposure occurred years before the onset of symptoms.
Dietary history
A history of malnutrition or adherence to a specialised diet, such as a low-protein, vegetarian, or vegan diet, may lead to specific nutritional deficiencies.
Family history
A positive family history of paraesthesias or sensory impairment may indicate an inherited neuropathy or an inborn error of metabolism. A family history of acquired diseases such as compression neuropathies, autoimmune disease, cancer, amyloidosis, diabetes mellitus, multiple sclerosis, or migraine may also be present.
Social/occupational history
A history of smoking should raise suspicion of a paraneoplastic syndrome. A history of occupational, environmental, or recreational exposure to heavy metals may indicate heavy metal toxicity. The presence of risk factors for HIV exposure or infection should prompt suspicion of HIV neuropathy.
Psychiatric history
Panic attacks with hyperventilation are very common causes of paraesthesias. Patients report associated symptoms of anxiety or panic including an overwhelming sense of impending doom. Physical symptoms include chest pain, palpitations, and shortness of breath with perioral and bilateral paraesthesias in the hands and feet and an associated carpopedal spasm. More rarely, a history of psychiatric symptoms and/or psychological or physical trauma may indicate a conversion or somatic symptom disorder.
General examination
Initial
Fever may indicate infection. Lymphadenopathy may indicate infection (HIV, herpes simplex, Lyme disease) or systemic inflammatory disease. Diabetes mellitus or cardiovascular disease should be considered if the patient is overweight or obese. Weight loss may be a sign of underlying malignancy. Vascular assessment of the lower limb may reveal reduced or absent peripheral pulses in peripheral vascular disease.
Head/eyes/ears/nose/throat
Carotid bruits should prompt suspicion of stroke. Dry oral mucous membranes may indicate Sjogren's syndrome.
Skin
Purpura or rash suggests infection or vasculitis. Blistering in a dermatomal pattern in the area of the patient's paraesthesias indicates shingles. Skin colour changes or thickening of the skin may indicate monoclonal protein production, sarcoidosis, or an inborn error of metabolism. A shiny, oily, or scaly appearance of the legs and loss of hair over the dorsum of the foot may indicate ischaemia due to peripheral vascular disease.
Bone and joints
High arched feet (pes cavus) and/or hammer-toe deformities may indicate an inherited peripheral neuropathy. Characteristic joint deformities may be present with underlying rheumatological conditions. Scoliosis may indicate osteoporosis, which may predispose the patient to spinal compression fractures. Tenderness to palpation along the spinal column suggests a radiculopathy due to spinal disease.
Rectal examination
Should be performed to assess anal tone. A decrease in anal tone indicates a radiculopathy or spinal cord lesion and requires urgent assessment.
Funduscopy
May reveal diabetic retinopathy in a diabetic patient.
Neurological examination
Sensory system
The neurological examination should focus in detail on the areas that correspond to the patient's symptoms, but a general neurological examination should also be carried out to identify additional clues to the underlying diagnosis. The first step is to examine the sensory modalities in all four extremities, with additional testing in the areas where the patient is reporting the paraesthesias.
Light touch is tested with a cotton wisp. Temperature is tested using a cold or warm stimulus. Pinprick is tested using the sharp end of a safety pin or other standard stimulus-producing tool, which should be single-use only and disposed of appropriately after being used on each patient. Joint position sense is tested using the distal interphalangeal (DIP) joints of the big toe or middle finger. Vibration is tested using a 128-Hz tuning fork placed at the DIP joint of the big toe or middle finger. The examination may reveal one of the following patterns.
Sensory loss in the distribution of a single peripheral nerve indicates a peripheral mononeuropathy. Palpation of an individual peripheral nerve or specific provocative testing (such as Tinel's or Phalen's sign) may elicit the paraesthesias, indicating a nerve entrapment syndrome.
Sensory loss in the distribution of multiple peripheral nerves indicates multiple mononeuropathies or a plexopathy.
Symmetric 'glove and stocking' distribution of sensory loss indicates a peripheral polyneuropathy.
Sensory loss in a dermatomal distribution indicates a radiculopathy. The corresponding spinal nerve is also affected and the distribution of the spinal nerve should also be tested for sensory loss.
A sensory spinal level on the trunk suggests spinal cord pathology.
Unilateral sensory loss affecting the face or extremities indicates a spinal cord or brain lesion.
Motor system
The presence of muscle atrophy should be noted. Weakness or atrophy in the same distribution as the sensory loss indicates a mixed peripheral sensori-motor neuropathy (if the peripheral nerve distribution is affected), involvement of a spinal root (if a dermatome and myotome are affected), or spinal cord or brain pathology. Loss of deep tendon reflexes indicates a peripheral lesion. Increased deep tendon reflexes, upgoing plantar reflex, and muscle spasticity indicate a spinal cord or brain lesion.
Gait
Abnormalities to note include a wide-based or ataxic or unsteady gait, a foot drop or difficulty walking due to weakness, or spasticity of the lower limbs. The patient should be asked to stand with a narrow base and then close their eyes and maintain their balance (Romberg's test); this is a test of dorsal column function. Asking the patient to walk on their tiptoes and on their heels allows assessment of the strength of the calf muscles and the foot and toe extensors. Tandem gait (walking heel-to-toe in a straight line) is a very sensitive test of general gait stability.
Gait imbalance and incoordination usually indicate a sensory ataxia, which is seen in vitamin B12, vitamin E, and copper deficiencies; severe diabetic neuropathy; alcoholic neuropathy; neurosyphilis; CIDP; paraneoplastic sensory neuropathy; Sjogren's syndrome; and some inborn errors of metabolism. Gait abnormalities due to central nervous system (CNS) disease can be seen in multiple sclerosis or acute disseminated demyelinating encephalomyelitis. A clumsy gait may be related to foot drop, as seen in fibular (peroneal) neuropathy or Charcot-Marie-Tooth disease. Patients with spinal cord compression may have difficulty walking due to spasticity and/or muscle weakness in the lower extremities.
Cerebellar function
Should be assessed by testing the patient's ability to perform heel-to-shin and finger-to-nose-to-finger manoeuvres. This helps to detect cerebellar pathology in patients with alcohol abuse, paraneoplastic disease, or CNS disease.
Cranial nerve assessment
Abnormalities may occur in a range of conditions including multiple sclerosis (usually affecting the optic nerve), trigeminal neuropathy, fish poisoning, Lyme disease, Sjogren's syndrome, monoclonal protein production, and some inborn errors of metabolism.
Assessment of cranial nerve function should therefore be performed as part of the assessment: the pupils should be equal, round, and reactive to light stimulation. Optic disc pallor on funduscopic examination may indicate optic atrophy due to multiple sclerosis. Facial sensation to light touch, temperature, and pinprick stimulation should be tested. If a sensory abnormality is found on the face, additional testing of corneal reflexes with a wisp of cotton should be performed to further confirm trigeminal dysfunction on the affected side. Facial, trapezius, and sternocleidomastoid muscle strength and symmetry should be assessed.
Investigations for peripheral nerve involvement
Further diagnostic testing is guided by the history and physical examination findings, and consists of laboratory tests to identify the underlying cause and electrophysiological studies to confirm and characterise the type of peripheral neuropathy.
Metabolic or endocrine tests
One of the most important tests is a 2-hour glucose tolerance test (or at a minimum, a fasting glucose or HbA1c test). HbA1c is useful to detect poor glycaemic control in patients with known diabetes mellitus and may be elevated. Serum calcium, vitamin D, and PTH levels should be measured if hypocalcaemia is suspected. Serum magnesium levels should also be measured in these patients and may be reduced; hypocalcaemia occurring in the context of hypomagnesaemia does not resolve until magnesium levels are corrected. An elevated serum thyroid-stimulating hormone and a low free thyroxine suggest hypothyroidism.
Heavy metals, toxin, or drug levels
Tests should be ordered if there is a history of potential exposure to a heavy metal. Whole blood lead levels are elevated in lead toxicity, and 24-hour urinary arsenic, mercury, or thallium levels are elevated with the corresponding heavy metal toxicity. Serum ciguatera toxin and saxitoxin levels should be measured if fish poisoning is suspected. Urine hexanediol and hexenol levels should be measured if hexane toxicity is suspected.
There is no specific laboratory test for alcoholic neuropathy, but liver function tests should be measured in patients with a history of alcoholism and typically show elevated gamma-GT, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) (with AST>ALT).
Drug-induced neuropathy should resolve once the causative medications are discontinued.
Vitamin levels
Vitamins B12, B1, B6, and E and methylmalonic acid levels should be measured in patients who are at risk for these deficiencies. Symptoms should resolve if a therapeutic trial of supplementation is instigated. Transketolase activity in whole blood or red blood cells may also be used to test for vitamin B1 deficiency, and typically increases after the addition of thiamine. Copper levels can be measured to exclude copper deficiency if this is suspected. Zinc levels may be elevated if the deficiency is produced by zinc toxicity. Serum vitamin B12 may also be decreased. Excess vitamin B6 supplementation (i.e., >50 mg/day) can also cause sensory peripheral neuropathy. Checking blood levels of vitamin B6 can help to determine if deficiency or toxicity is present.
Lumbar puncture (LP)
If patients are suspected to have AIDP based on the clinical features, an urgent LP with cerebrospinal fluid (CSF) examination is required, and shows the characteristic finding of albuminocytological dissociation (elevated CSF protein with normal CSF cell counts). Patients with suspected CIDP or CISP, based on the clinical pattern, also require LP with CSF examination, which shows albuminocytological dissociation. In patients with peripheral neuropathy after bariatric surgery, a lumbar puncture will show increased protein with a normal white cell count.
Diagnostic lumbar puncture in adults: animated demonstrationHow to perform a diagnostic lumbar puncture in adults. Includes a discussion of patient positioning, choice of needle, and measurement of opening and closing pressure.
Infective markers
HIV neuropathy should be suspected in known HIV-positive patients. An HIV antibody test should be performed if HIV infection is suspected.
Human T-lymphotropic virus (HTLV) type I and II antibodies should be tested for in at-risk individuals.
A skin smear of skin lesions should be performed in patients with suspected leprosy; Mycobacterium leprae is isolated.
Viral cultures or PCR of skin lesions confirm HSV infection in patients with typical lesions. Serum anti-HSV-1 and HSV-2 antibodies should be measured to identify the subtype of the virus.
Shingles, caused by varicella zoster infection, is a clinical diagnosis, but a polymerase chain reaction (PCR) can be used if the diagnosis is in doubt.
Serum rapid plasma reagin (RPR) test or Venereal Disease Research Laboratory (VDRL) test should be performed if neurosyphilis is suspected. If serology is negative and neurosyphilis remains a concern, CSF analysis for VDRL must be performed before the diagnosis can be excluded or confirmed.
Serum Lyme enzyme immunoassay (EIA) or immunofluorescence assay (IFA) should be performed to test for Lyme disease if this is suspected.[65]
Inflammatory markers
If patients present with painful neuropathy, especially mononeuritis multiplex, vasculitis markers should be measured. Antinuclear antibody, extractable nuclear antigens, anti-double-stranded DNA, antineutrophil cytoplasmic antibody, serum cryoglobulins, serum complement, and rheumatoid factor are useful markers of the underlying vasculitides.[34] The C-reactive protein (CRP) is often highly elevated. An FBC shows eosinophilia in Churg-Strauss syndrome, or a normocytic anaemia with leukocytosis and thrombocytosis in granulomatosis with polyangiitis (formerly known as Wegener's granulomatosis).
Anti-60 kDa Ro (SSA), anti-La (SSB) antibodies, and Schirmer's test should be performed in patients with suspected Sjogren's syndrome. Additional confirmatory tests include quantitative sweat and sensory testing, which show sensory and sympathetic nerve dysfunction and Rose-Bengal staining of the eye, showing a severe dry eye.
A diagnosis of sarcoidosis usually requires confirmation by tissue biopsy, and is usually already established in most patients. Angiotensin converting enzyme (ACE) levels and calcium may be high. Chest x-ray reveals hilar adenopathy or widening of the mediastinum. Tuberculin skin test should be negative.
Other laboratory tests
Patients with CIDP (diagnosed by clinical findings and LP) should have tests for anti-myelin-associated protein and anti-ganglioside antibodies (specifically anti-GM1). Serum protein electrophoresis with immunofixation identifies the subtype.
Serum and urine electrophoresis with immunofixation should be performed if monoclonal protein production is suspected. If positive, further tests for the specific underlying disease should be undertaken and include serum amyloid free light chains (positive in amyloidosis), serum cryoglobulins and hepatitis C antibodies (positive in cryoglobulinaemia), and serum beta-2 microglobulin (increased in Waldenstrom's macroglobulinaemia).
Inherited neuropathies should be suspected if there is a strong family history, an insidious onset of painless symptoms, or associated deformities such as hammer-toe deformity or pes cavus, or if an acquired cause is not identified. Genetic testing identifies the underlying causative mutation. A clinical picture screen can be used to identify patients with inborn errors of metabolism.[66]
In distal symmetric polyneuropathy, additional testing is determined by the suspected underlying aetiologies, including hereditary, infectious, toxic, nutritional, and inflammatory conditions.[13][14]
Peripheral vascular perfusion studies
If peripheral vascular disease is suspected as the cause, patients require an assessment of peripheral vascular perfusion that includes ankle brachial index ≤0.90 with a gradient of >20 mmHg between adjacent segments on segmental pressure examination; duplex ultrasound showing a peak systolic velocity ratio >2.0, and subsequent angiography revealing stenosis of the affected artery.
Electromyography and nerve conduction studies
Diabetic neuropathy, hypocalcaemia, vitamin deficiencies, drug toxicity, and minor infections such as shingles or HSV can usually be diagnosed clinically or with laboratory testing. All other peripheral neuropathies may require EMG with nerve conduction studies to confirm and characterise the neuropathy.
EMG includes nerve conduction studies and needle examination of selected muscles and should be directed at nerves and muscles whose involvement is suspected based on the clinical examination. EMG is able to characterise the type of peripheral nerve abnormality by its electrophysiological features (e.g., an axonal or demyelinating neuropathy). It provides a definitive diagnosis of nerve entrapment syndromes and can also identify more proximal lesions such as radiculopathies or plexopathies.[Figure caption and citation for the preceding image starts]: Nerve conduction testing of the lower legCreated by the BMJ Group [Citation ends].
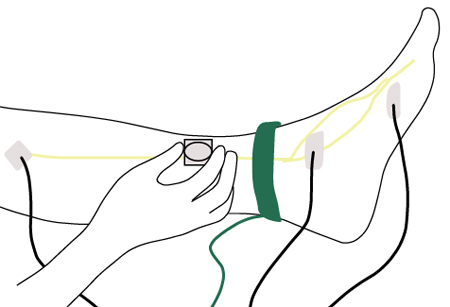
EMG helps distinguish AIDP and CIDP from CISP. AIDP and CIDP produce sensori-motor peripheral neuropathy, whereas the EMG in CISP is normal.
Small-fibre sensory neuropathy (which typically produces burning paraesthesias) cannot be excluded by standard EMG and must be investigated using quantitative sensory or sudomotor testing, or skin biopsy to quantify intra-epidermal nerve fibre densities.
Further investigations
May be considered based on the associated symptoms and EMG findings.
Patients with nerve entrapment syndromes may require further imaging to identify the site of the compression, identify causative lesions, and assess the patient prior to surgery. Imaging modalities used include ultrasound of the wrist or elbow and magnetic resonance imaging (MRI) of the elbow, knee, or ankle.
Patients with suspected CISP (clinical features, albuminocytological dissociation, normal EMG) require somatosensory-evoked potentials from the upper and lower extremities that show delayed conduction of potentials through the spinal cord due to demyelination of the dorsal sensory roots. An MRI of the spine, done with and without contrast administration, may help to confirm the site of the lesion by virtue of enlarged or enhancing dorsal roots, or both.
Paraneoplastic antibodies (particularly anti-Hu) can be tested in patients with high levels of suspicion. If patients have positive paraneoplastic antibodies, thorough investigation is required to identify the source of the primary tumour. This includes a mammogram in female patients; a computed tomographic (CT) scan of the chest, abdomen, and pelvis; and a whole-body PET scan. If the initial CT scan is negative, it should be repeated every 3 to 6 months until a cause is found for at least the first 2 years after diagnosis. Whole-body PET scan should be repeated annually if other screening tests for cancer are negative.
If patients have autonomic symptoms, including orthostatic dizziness and sweating abnormalities, specific autonomic function testing is helpful. This may be considered in patients with possible inherited sensory and autonomic neuropathy, in patients with paraesthesias and autonomic dysfunction in Sjogren's syndrome, or in those with distal symmetric polyneuropathy, or polyneuropathy after bariatric surgery.[36]
Peripheral nerve biopsy may be considered for patients with peripheral neuropathy that is suspected to be due to an inflammatory cause such as vasculitis, monoclonal protein deposition, sarcoidosis, distal symmetric polyneuropathy, polyneuropathy after bariatric surgery, or if the aetiology is not readily determined by non-invasive methods.[13][14][30][55][67][68] The nerve biopsy should be done in a nerve that is involved in the disease process. Usually the sural nerve is biopsied just proximal to the ankle, but other cutaneous nerves can be used.[68] The risks of the procedure are very small, but include bleeding, infection, poor wound healing, and neuroma formation at the site of the biopsy. Almost all patients experience neuropathic pain at the site of the biopsy, which typically persists for a few days to a few weeks, and permanent numbness in the distribution of the biopsied nerve distal to the biopsy site.
In distal symmetric polyneuropathy, a skin biopsy to determine epidermal nerve fibre density may be an alternative to peripheral nerve biopsy in selected cases.[13][14]
Plexopathy or radiculopathy
Plexopathy
An MRI of the plexus with contrast is important to confirm possible nerve compression by a mass lesion (such as an invading malignancy) or a patchy inflammatory process.
Radiculopathy or ganglionopathy
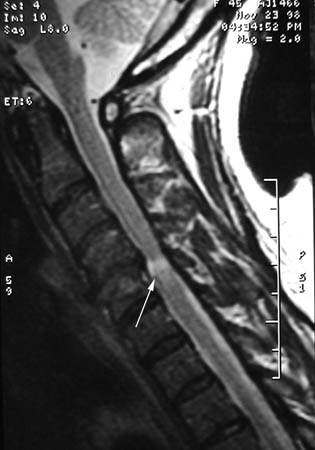
MRI with contrast of the suspected spinal level (cervical, thoracic, lumbosacral) is required to search for evidence of disc herniation, facet arthropathy, spondylosis, spinal canal stenosis, or neuroforaminal stenosis. Anatomical lesions identified by imaging should correlate with the dermatomal distribution of the paraesthesias. The MRI may also show abnormal enhancement or enlargement of nerve roots, which may occur in CISP or lymphomatous infiltration of the nerve roots in the spinal canal. If the nerve roots are enhancing or enlarged, LP with CSF examination should be performed and reveals abnormal cytology in lymphoma or albuminocytological dissociation in CISP.[Figure caption and citation for the preceding image starts]: A single level of spinal cord compression with T2 changes, on cervical sagittal T2 sequence in the presence of symptomatic cervical spondylotic myelopathy (CSM)From the collection of Professor Dennis Turner, Duke University Medical Center; used with permission [Citation ends].
 [Figure caption and citation for the preceding image starts]: Axial T2-weighted MRI with broad-based lumbar disc herniation predominantly towards the right sideFrom the collection of Alexios G. Carayannopoulos, Lahey Clinic; used with permission [Citation ends].
[Figure caption and citation for the preceding image starts]: Axial T2-weighted MRI with broad-based lumbar disc herniation predominantly towards the right sideFrom the collection of Alexios G. Carayannopoulos, Lahey Clinic; used with permission [Citation ends].
Spinal cord involvement
MRI of total spine with contrast
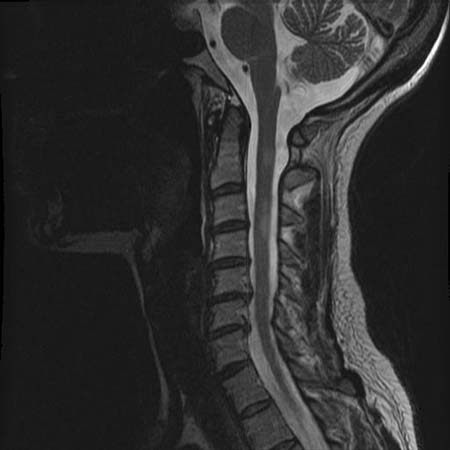
This is the image modality of choice in most patients. May show disc displacement, epidural enhancement, a mass effect, or a T2 cord signal if spinal cord compression is present. MRI may also reveal characteristic lesions of multiple sclerosis or transverse myelitis. Enlargement and abnormal enhancement of spinal nerve roots may be present in patients with sarcoidosis.[Figure caption and citation for the preceding image starts]: Sagittal T2-weighted MRI showing MS-related myelitis lesionFrom the collection of Dean M. Wingerchuk, Mayo Clinic; used with permission [Citation ends].
Spine x-ray
This is the first test if there is a history of trauma or a compression fracture is suspected. May reveal decreased disc space height (disc compression), loss of bony detail (tumour, infection), misalignment of vertebral elements (trauma), or loss of endplate definition (infection).
Lumbar puncture
CSF examination should be performed to exclude infection and demonstrate characteristic findings of multiple sclerosis and transverse myelitis (elevated protein and oligoclonal bands, and increased CSF IgG index).
Diagnostic lumbar puncture in adults: animated demonstrationHow to perform a diagnostic lumbar puncture in adults. Includes a discussion of patient positioning, choice of needle, and measurement of opening and closing pressure.
Infective markers
If infectious causes are suspected, testing for syphilis (tabes dorsalis) or HIV (HIV myelopathy) should be performed.
Brainstem or brain involvement
Brain imaging
CT brain should be considered in patients with an acute onset of numbness in the face or extremities and clinical features suggestive of a stroke to exclude haemorrhage. This should be followed with an MRI to identify ischaemic stroke. An MR angiogram of the Circle of Willis and neck may demonstrate focal stenosis of medium- to-large-diameter vessels, suggesting a possible source of emboli.
An MRI brain should be considered in patients with suspected multiple sclerosis or acute demyelinating encephalomyelitis, and may reveal characteristic demyelinating lesions. In acute demyelinating encephalomyelitis, the lesions are multi-focal and may contain haemorrhages. [Figure caption and citation for the preceding image starts]: Sagittal FLAIR images with typical MS lesionsFrom the collection of Lael A. Stone, Cleveland Clinic Foundation; used with permission [Citation ends].

Lumbar puncture
CSF examination reveals the characteristic features of elevated protein and oligoclonal bands and increased CSF IgG index in patients with multiple sclerosis or acute demyelinating encephalomyelitis, and should be performed if these conditions are suspected.
Diagnostic lumbar puncture in adults: animated demonstrationHow to perform a diagnostic lumbar puncture in adults. Includes a discussion of patient positioning, choice of needle, and measurement of opening and closing pressure.
Visual-evoked potentials
May show delayed conduction in patients with multiple sclerosis associated with optic neuritis.
Electroencephalogram (EEG)
Should be considered in patients with a focal sensory seizure to look for interictal epileptogenic discharges in the suspected area of origin in the cortex.
Use of this content is subject to our disclaimer
