Frailty as an upstream target for intervention: A unifying approach to intervening in the trajectories of health, function, and disease in late life
Abstract
This editorial comments on the article by Deiner et al. in this issue.
In Deiner et al.'s analysis1 of data from The Successful Aging after Elective Surgery (SAGES) Study,2 pre-operative frailty measured by a deficit-accumulation index3 was shown to be associated with nearly twice the risk of post-operative delirium, an association that persisted after controlling for pre-operative cognitive status. This well-constructed study examined both the frailty phenotype4 and the deficit-accumulation frailty index,3, 5 leveraging a plethora of robust outcome data in this surgical cohort study.
For those engaged in the clinical care of older adults, it will come as no surprise that frailty predisposes to delirium. Almost by definition, bad things happen to those with frailty—their lack of reserve in the face of stressors may produce any number of adverse post-operative events. But how and why and what can be done about it?
Delirium, and other geriatric syndromes, emerge clinically when predisposing factors render individuals vulnerable upon exposure to precipitating factors and insults6 (Figure 1). A wealth of research has now shown that the frailty construct is extremely useful as a representation of such baseline vulnerability in older adults. Irrespective of whether frailty is operationalized as a state (e.g., Fried Frailty Phenotype)7 or as a process (e.g., Rockwood Stochastic Deficit Accumulation Frailty Index),8, 9 frailty shows consistent associations with various chronic diseases, declines in function, disability, hospitalization, institutionalization, and death. Nonetheless, it is becoming clear that these two different approaches to frailty capture complementary facets of the risk factors shared by varied geriatric syndromes such as delirium, and other common conditions and diseases of late life for which aging itself represents the greatest proportion of overall risk.10
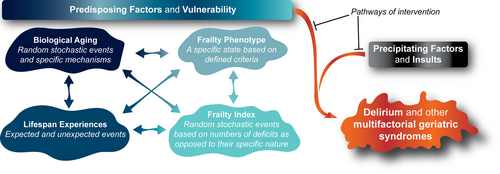
Of course, one needs to be careful not to engage in flawed circular arguments whereby a clinical measure is used to define the existence of a condition or process (e.g., frailty), and then the very same condition or process (e.g., frailty) is also used to predict that clinical measure. In fact, as outlined below, we propose that when examined together these complementary methodologies used to measure frailty may allow for a deeper understanding of the multifaceted contributions of potentially modifiable risk factors. Such relevant predisposing risk factors range from modifiable aspects of biological aging to potentially preventable clinical stressors involved in the clinical trajectories leading to health, function, and varied chronic diseases and geriatric syndromes.
As outlined in the Geroscience hypothesis, strong evidence is emerging that root biological causes of aging contribute to the onset and progression of different geriatric syndromes and chronic diseases of late life by rendering a person's brain and body physiologically more vulnerable and less capable of responding to new stressors. An impediment to greater progress from convincing animal model studies to humans has been the fact that hallmarks of biological aging that drive frailty and decreased resilience11 are difficult to evaluate and measure in clinical settings12 unless and until they become manifested in terms of evident cumulative deficits and observable phenotypes of geriatric syndromes. Typically, many such underlying processes and vulnerabilities are not noticed under baseline conditions, yet they often become apparent if and when the individual is challenged with a stressor or a significant precipitating event.
In addition to the involvement of specific predetermined biological pathways, stochastic (random) events also play an important role in biological aging (Figure 1). Since all of these processes collectively contribute to biological aging and increased vulnerability, it is helpful to view and measure frailty through the lens of both the Frailty Index and the Frailty Phenotype. In spite of a growing emphasis on the importance of specific biological hallmarks of aging, it has also become clear that biological aging can be both accelerated and attenuated by targeting any one of the individual hallmarks.
With all of the above considerations in mind, the Frailty Index may be viewed as being reflective of more stochastic upstream biological drivers whereby numbers of seemingly random deficits are more important than the existence of any specific deficit. In contrast, the Frailty Phenotype is obviously much more appropriate for the detection of a specific state which is defined by the presence or absence of a small number of precisely defined criteria. Thus, the Frailty Index may offer insights into those upstream shared risk factors including biological aging that predispose and drive the downstream emergence of common geriatric syndromes, including the Frailty Phenotype and delirium, amongst others (Figure 1).10
“The Frailty Index may be viewed as being reflective of more stochastic upstream biological drivers whereby numbers of seemingly random deficits are more important than the existence of any specific deficit. In contrast, the Frailty Phenotype is obviously much more appropriate for the detection of a specific state which is defined by the presence or absence of a small number of precisely defined criteria.”
Having the same term (frailty) appear in two completely different portions of the process has posed some challenges and even confusion over the years. However, efforts to measure, further refine, and correctly utilize both of these distinct constructs will in our view help advance the field. Moreover, viewing the entire process as an inevitable blend of risks and processes involving specific age-associated mechanisms and pathways, together with the onset and acquisition of stochastic events may also help conceptualize current clinical care and interventions. Ultimately, this approach offers the potential to alter trajectories of clinical outcomes involving health, function, or varied disease states. Based on these considerations, this state of vulnerability may ultimately manifest itself clinically following a precipitating stressor event. Exposure to a stressor or precipitant event could contribute to the emergence of one or more of a variety of well-described observable geriatric syndromes, with the resulting constellation of clinical findings depending on the timing and nature of the stressor and on individual-specific pre-existing deficits (Figure 1).
Emergent impairments in cognition and physical function typically represent the final common pathway, often peppered with one or more pre-existing organ-based diseases. For example, an older adult with frailty undergoing elective surgery may emerge with delirium, mobility disability, and atrial fibrillation with rapid ventricular response, all superimposed upon other pre-existing chronic conditions—with each individual patient expressing a unique constellation of clinical features that resulted from interactions between aging-associated deficits and precipitating events. However, in contrast to the highly integrative systems-based multidisciplinary nature of comprehensive geriatric care, research efforts seeking greater specificity and precision have sometimes established false barriers between brain and body. While some have envisioned cognition, physical function, or diseases of aging as the single most important dimension of clinically modifiable aging, Deiner et al. demonstrate, through the inclusion of both constructs of frailty, and through the association between a “physical” and a “cognitive” construct, that the next stage of aging research must focus on what unites, as opposed to dividing our understanding of aging. Only once we are able to view and accept aging as a complex interaction between biological drivers, deficits, vulnerabilities, precipitating events, and outcomes may we be able to successfully intervene in the trajectory toward health, function, and independence. In our view, this multifaceted and multidisciplinary approach is needed if we are to deliver on the promise of increased health span.
By studying those living with and without frailty, and quantifying and qualifying their deficits, researchers may help overcome the barriers to clinical translation. This approach will establish links between biological hallmarks of aging and age-associated deficits, and focus on the path from those deficits to their clinical expression8, 13 (i.e., geriatric syndromes like dementia, incontinence, falls, delirium, or chronic diseases of aging like diabetes, chronic kidney disease, etc.). Moreover, such an approach could ultimately offer opportunities for actionable clinical decisions in the care of frail older adults. For example, high quality randomized controlled trials have demonstrated the benefits of multicomponent interventions targeting risk factors as varied as cognitive impairment, sleep deprivation, immobility, visual impairment, hearing impairment, and dehydration in preventing the number and duration of delirium episodes in hospitalized older patients.14 Further study of precipitating factors (trauma, infection, surgery, polypharmacy, etc.) in frail and non-frail populations may identify additional potentially preventable risk factors as targets for future intervention efforts (Figure 1). Moreover, use of gerotherapeutics shown to slow biological aging which include not only medications,15 but also behavioral interventions like exercise or improved sleep,16 may help diminish the risk of delirium by attenuating the underlying predisposing drivers (Figure 1). This type of a multicomponent and multidisciplinary approach allows for a more complete model of the pathways from vulnerability to adverse events while highlighting novel and even unexpected opportunities to intervene multiple emergent syndromes before they manifest as a disability.
This transformational approach recognizes that multicomponent pharmacological, behavioral, or lifestyle interventions targeting biological aging upstream of disability16 may help insulate older adults from the cascade linking biological aging to deficits to disability. If only a small trigger is needed to unbalance homeostasis in a highly vulnerable individual, while a major event may be withstood by someone more robust—how may we prevent vulnerability (i.e., frailty) or bolster those with frailty? While we may never have one unifying cause of accelerated aging, targeting the handful of recognized hallmarks may aid all older adults, as a rising tide is said to lift all ships. Interventions may also need to be adjusted up or down to meet the needs of the individual. SMART designs17 and other approaches toward Precision Gerontology exemplify an allowance for some adaptation to meet the profile of the individual, an increasing part of both pragmatic and implementation science designs.
However, to achieve these ambitious goals, research in adults past middle age needs to consistently include frailty status, while at the same time developing a Geroscience-guided perspective including the measurement of biological age using a growing number of biomarkers (Table 1).18 Not only may these individuals be more likely to develop the outcome of interest, but also, they may be more likely to benefit from the intervention. Lumping all older adults together via a traditional “one size fits all” approach to evidence-based medicine is unlikely to allow us to see signal through noise.
| Identify and categorize risk when enrolling populations |
| Stratify research enrollment by frailty status |
| Consider composite outcomes of geriatric syndromes (e.g., falls, delirium, phenotypic frailty) |
| Explore and interrogate the inter-relationship of cognition, function, and physiological change |
| Where possible, include hallmarks of accelerated biological aging to prepare for future intervention studies |
For clinical work, it remains vitally important to minimize the impact of precipitants to avoid further declines in function and independence. This study, and similar work, highlight how a frailty metric may identify a subset of the most vulnerable individuals for whom to target preventive measures. Most importantly, it is time to (a) categorize risk when enrolling populations and stratify by frailty; (b) consider composite outcomes for geriatric syndromes such as falls, delirium, and even phenotypic frailty, as a final common pathway after a precipitating event; and (c) further interrogate the inter-relationship of cognition, function, and physiological change as all part of soon-to-be modifiable hallmarks of accelerated biological aging.
AUTHOR CONTRIBUTIONS
Both authors contributed equally.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest.
FINANCIAL DISCLOSURE
NIA (P30AG067988).
SPONSOR'S ROLE
A sponsor did not play any role in this article.