Article Text
Abstract
Background and aims: Activation of corticotropin releasing factor 1 (CRF1) receptors is involved in stress related responses and visceral pain, while activation of CRF2 receptors dampens the endocrine and some behavioural stress responses. We hypothesised that CRF2 receptor activation may influence visceral pain induced by colorectal distension (CRD) in conscious rats, and assessed the possible sites and mechanisms of action.
Methods: Male Sprague-Dawley rats were exposed to CRDs (60 mm Hg, 10 minutes twice, with a 10 minute rest interval). Visceromotor responses (VMR) were measured by electromyography or visual observation. Spinal (L6–S1) extracellular signal regulated kinase 1/2 (ERK 1/2) activation following in vivo CRD and CRF2 receptor gene expression in the T13–S1 dorsal root ganglia (DRG) and spinal cord were determined. Inferior splanchnic afferent (ISA) activity to CRD (0.4 ml, 20 seconds) was assessed by electrophysiological recording in an in vitro ISA nerve-inferior mesenteric artery (intra-arterial)-colorectal preparation.
Results: In controls, VMR to the second CRD was mean 31 (SEM 4)% higher than that of the first (p<0.05). The selective CRF2 agonist, human urocortin 2 (hUcn 2, at 10 and 20 μg/kg), injected intravenous after the first distension, prevented sensitisation and reduced the second response by 8 (1)% and 30 (5)% (p<0.05) compared with the first response, respectively. RT-PCR detected CRF2 receptor gene expression in the DRG and spinal cord. CRD (60 mm Hg for 10 minutes) induced phosphorylation of ERK 1/2 in neurones of lumbosacral laminae I and IIo and the response was dampened by intravenous hUcn 2. CRD, in vitro, induced robust ISA spike activity that was dose dependently blunted by hUcn 2 (1–3 μg, intra-arterially). The CRF2 receptor antagonist, astressin2-B (200 μg/kg subcutaneously or 20 μg intra-arterially) blocked the hUcn 2 inhibitory effects in vivo and in vitro.
Conclusions: Peripheral injection of hUcn 2 blunts CRD induced visceral pain, colonic afferent, and spinal L6-S1 ERK 1/2 activity through CRF2 receptor activation in rats.
- AUC, area under the curve
- CRD, colorectal distension
- CRF, corticotropin releasing factor
- DRG, dorsal root ganglia
- EMG, electromyogram
- hUcn 2, human urocortin 2
- IBS, irritable bowel syndrome
- IML, intermediolateral column
- ISA, inferior splanchnic afferent
- MAPK, mitogen activated protein kinase
- pERK, phosphorylated extracellular signal regulated kinase
- RF, receptive field
- RT-PCR, reverse transcriptase-polymerase chain reaction
- VMR, visceromotor response
- IIo, laminae II outer layer
- corticotrophin releasing factor
- urocortin 2
- astressin2-B
- visceral pain
- colon
- ERK 1/2
- inferior splanchnic afferents
- colorectal distension
Statistics from Altmetric.com
- AUC, area under the curve
- CRD, colorectal distension
- CRF, corticotropin releasing factor
- DRG, dorsal root ganglia
- EMG, electromyogram
- hUcn 2, human urocortin 2
- IBS, irritable bowel syndrome
- IML, intermediolateral column
- ISA, inferior splanchnic afferent
- MAPK, mitogen activated protein kinase
- pERK, phosphorylated extracellular signal regulated kinase
- RF, receptive field
- RT-PCR, reverse transcriptase-polymerase chain reaction
- VMR, visceromotor response
- IIo, laminae II outer layer
- corticotrophin releasing factor
- urocortin 2
- astressin2-B
- visceral pain
- colon
- ERK 1/2
- inferior splanchnic afferents
- colorectal distension
Activation of corticotropin releasing factor (CRF) signalling pathways plays a key role in the body’s response to stress.1–3 In addition to CRF, the CRF family encompasses three novel CRF related mammalian ligands, urocortin 1 (Ucn 1), Ucn 2, and Ucn 3.4,5 CRF ligands display distinct affinities to CRF receptor subtype 1 (CRF1) and/or CRF2. CRF has preferential affinity for CRF1, Ucn 1 binds with equal high affinity to both CRF receptors, while Ucn 2 and Ucn 3 exhibit high selectivity towards CRF2 receptors.4,5 The availability of CRF1 antagonists that cross the blood-brain barrier and recent development of selective peptide CRF2 receptor antagonists, such as antisauvagine-30 and astressin2-B,6,7 have spurred interest in defining the role of CRF receptor subtypes in stress responses.2,3,8
In experimental animals, CRF1 is the main receptor implicated in stress related activation of colonic motility, induction of watery diarrhoea, and hypersensitivity to colorectal distension (CRD).9 Likewise, in healthy subjects, intravenous administration of ovine CRF, a preferential CRF1 receptor agonist,5 mimicked stress related visceral responses.10,11 The peptide lowered the threshold for sensation of the urge to defecate and of discomfort to CRD,10 and increased the colonic motility index.11 A recent study also indicated that intravenous injection of the non-selective CRF receptor antagonist, α-helical CRF12-41,5 improved colonic motility and visceral pain induced by rectal electrical stimulation in irritable bowel syndrome (IBS) patients.12 These findings suggest the possible relevance of CRF1 receptor antagonists for the treatment of IBS.9,13
Regarding the role that CRF2 receptors play in mediating or modulating stress induced responses, recent reports put forward the concept that activation of CRF2 signalling pathways may be important to dampen stress sensitivity.8 CRF2 ligands reduce CRF1 mediated activation of the hypothalamic-pituitary-adrenal axis, energy expenditure, and some behavioural responses to stress.8,14 Activation of central CRF1 and peripheral CRF2 receptors also results in opposite changes in blood pressure.15–17
In the current study, we investigated whether the activation of CRF2 receptor, induced by peripheral administration of Ucn 2, modulates CRD induced visceral pain in vivo and in vitro and its possible sites and mechanisms of action. Visceromotor responses (VMR) were monitored in conscious rats by measuring external oblique abdominal muscle contractions to repeated isobaric CRD. The CRF2 receptor mediated action of hUcn 2 was investigated using the selective CRF2 antagonist, astressin2-B,7 and assessing CRF2 receptor gene expression in dorsal root ganglia (DRG) and spinal cord (T13–S1). Recent studies showed that spinal activation of extracellular signal regulated kinase (ERK) is involved in the sensitisation process induced by applying irritants into the mouse colon or rat hind paw.18,19 We therefore investigated whether such a spinal signalling pathway is recruited in CRD induced visceral pain and is modulated by hUcn 2, using immunohistochemical assessment of ERK phosphorylation in the lumbosacral segments of the spinal cord known to be activated by CRD.20 Lastly, using a novel in vitro rat distal colon-inferior splanchnic nerve/inferior mesenteric artery preparation and electrophysiological recording of inferior splanchnic afferent (ISA) nerve activity, we examined whether close intra-arterial injection of Ucn 2 modulates CRD induced activation of ISA discharges.
MATERIALS AND METHODS
Animals and compounds
Adult male Sprague-Dawley rats (Harlan, San Diego, California, USA) weighing 280–320 g were housed in group cages with free access to rat chow and tap water. Animals were quarantined under controlled conditions of temperature and humidity for at least one week. Experiments started between 9am and 10am in non-fasted rats unless otherwise stated. Protocols were approved by the Animal Care Committee of the Veteran Affairs Greater Los Angeles Healthcare System and the UCLA Animal Research Committee.
Human Ucn 2 and astressin2-B (Clayton Foundation Laboratories for Peptide Biology, Salk Institute, La Jolla, California, USA) were synthesised as described previously7,21 and stored in powder form at −80°C. hUcn 2 was weighed and dissolved in saline, and astressin2-B in distilled water just before use. Bradykinin (Sigma Chemical Co, St Louis, Missouri, USA) was dissolved in saline.
Colorectal distension and monitoring of visceral motor response
CRD in awake rats produces contractions of abdominal and hindlimb musculature. This VMR has been validated as a quantitative measure of visceral nociception.22
For electromyogram (EMG) recording of abdominal muscles, fasted rats under sodium pentobarbital anaesthesia (Nembutal 70 mg/kg intraperitoneally; Abbott Labs, North Chicago, Illinois, USA) were chronically implanted with electrodes (Teflon coated stainless steel; AstraZeneca, Mölndal, Sweden) by stitching them onto the external oblique musculature immediately superior to the inguinal ligament, as previously described.23 The cannula, housing the electrode leads, was externalised on the left side of the abdominal wall. Following surgery, rats were housed in pairs and allowed to recuperate for at least 10 days during which they were trained to the experimental conditions by placing them singly in Bollmann cages for three hours per day for three days before the study. On the day of the experiment, rats were briefly anaesthetised with isoflurane, and a 6 cm long plastic balloon tied around an Intramedic PE-100 tubing (Becton Dickinson, Franklin Lakes, New Jersey, USA) was inserted intra-anally with the distal end positioned 1 cm proximal to the anus. An intravenous catheter (Insyte Autoguard; Becton Dickinson) was also inserted into the tail vein. Rats were then placed in Bollmann cages, and after a 60 minute stabilisation period were submitted to isobaric CRD (60 mm Hg, 10 minutes twice, with a 10 minute rest interval) using a pressure control device (AstraZeneca). Tonic CRD applied for 3–10 minutes in noxious and non-noxious ranges has been used previously to investigate mediators involved in colonic sensitisation.24,25
For VMR measurements, the externalised cannula was connected to a custom made EMG amplifier assembled to a Pentium class computer running the LabView (National Instruments, Dallas, Texas, USA) based proprietary software program for data acquisition (AstraZeneca).26 EMG signals were amplified, filtered (×10000, 300–5000 Hz), digitised, and rectified as detailed previously.23,26 Basal area under the curve (AUC) of contraction of the average peak EMG amplitude (V×s) was calculated as the area under the rectified EMG signal trace for the 10 minute period immediately preceding each 10 minute CRD. The AUC values of the EMG during the first and second distensions were computed and basal AUC subtracted to obtain the net AUC in response to CRD.
In vitro ISA nerve-colorectal preparation and recording
Fasted rats were exsanguinated by decapitation and the in vitro colorectal-ISA nerve preparation was isolated. This includes the proximal rectal segment (approximately 2 cm) and distal colon (about 3 cm, proximal portion of the isolated segment) with the attached inferior mesenteric artery, the lumbar colonic nerve, inferior mesenteric ganglion, and ISA nerves along with the intermesenteric nerve. The pelvic and hypogastric nerves were not included in the preparation. The isolated segment was transferred to the main chamber of a Sylgard coated (Dow Corning, Midland, Michigan, USA) organ bath that was perfused continuously with oxygenated Ringer’s solution at a 2.0–2.5 ml/min flow rate, as in previous studies.27 A 20 cm long PE-10 catheter (Becton Dickinson) was inserted into the inferior mesenteric artery for intra-arterial injections and secured in place by ligation. Subsequently, ISA nerves merging into the intermesenteric trunk were located and the proximal cut end of a thin nerve strand was placed on one electrode of a bipolar platinum wire electrode (diameter 30 μm) and a strand of connective tissue was attached to the other electrode.
Before each experiment, the most sensitive mechanoreceptive region of the colorectal preparation was searched for by gentle stimulation of the receptive field (RF) on the serosal side with a small paint brush. Then a balloon (∼9 mm diameter when distended with 0.4 ml air) attached to a PE-10 catheter was inserted through the oral end of the colorectal segment. The balloon was placed in the centre of the most mechanoreceptive region and a test CRD (20 seconds) for the nerve fibre spike activity conducted. The rationale for selecting short duration distension is based on the fact that the isolated colorectal preparation is devoid of the abdominal cavity pressure to counteract the intraluminal distension pressure and lacks the in vivo reflex mechanisms to adjust its compliance to tonic isobaric distensions.
The action potential of ISA fibres was preamplified (DAM-6, ×100, 100–10 kHz bandpass filter; World Precision Instruments, Sarasota, Florida, USA) and further amplified 300–750 times to give an action potential with a peak-peak amplitude of 1–5 V, which was displayed on a digital storage oscilloscope (model 2211; Tektronix Inc., Beaverton, Oregon, USA). Based on the amplitude and waveform, a particular unit can be traced off line to match the waveform of the unit by the use of the analysis module of the WAVEFORM software, as previously described.28 The response pattern of different units was analysed for the 20 second period before and during CRD.
Test substances were injected into the catheter positioned into the inferior mesenteric artery. The volumes of the dead space in the catheter (41.0 (1.5) μl; n = 10) and dead space plus vascular bed of the colorectal preparation (80.0 (1.7) μl, n = 3) were measured by injecting 0.02% toluidine blue intra-arterially. Based on this information, 0.1 ml was selected for intra-arterial injections that were performed over 20 seconds. Approximately 40 seconds later, the system was flushed with 0.1 ml of saline over 20 seconds. Because the organ bath was continuously perfused with oxygenated perfusate, compounds tested were rapidly flushed out of the system. The response magnitude after intra-arterial injection of vehicle or peptides per se was analysed by comparing the actual number of spikes/100 seconds before and after intra-arterial injections.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of CRF2 receptor gene expression
After decapitation of naïve rats (n = 6), both sides of DRGs and spinal cord tissue at T13–S1 levels of the spinal cord were collected in two separate experiments and stored at −80°C. Total RNAs were isolated with Qiagen mini RNeasy kit (Qiagen Inc., Valencia, California, USA). The first strand oligo-dT primed cDNA was synthesised from total RNA (500 ng of each pair of DRG and spinal cord from T13–S1 segments) by ThermoScript RNase H-minus reverse transcriptase at 55°C for one hour (Invitrogen, Carlsbad, California, USA). Oligonucleotide primers were designed to amplify a core region of CRF2 (sense 5′-TGC AAC ACG ACC TTG GAC CAG; antisense ATC ACA CGG CAG CTG TCT GCT PCR; 1120 bp). RT-PCR was performed using a Red-Taq DNA Polymerase System (Sigma). The housekeeping gene, acetic ribosomal protein (sense GTT GAA CAT CTC CCC CTT CTC; antisense ATG TCC TCA TCG GAT TCC TCC; 402 bp) was used as an internal control. The amplified PCR products were fractionated by electrophoresis in 1% agarose gel with ethidium bromide and detected under UV light. Gel images were recorded using a Kodak imaging system (1D Image Analysis, Eastman Kodak, Rochester, New York, USA). The identity of CRF2 fragment (1120 bp) was confirmed by DNA sequencing (Cycle Sequencing, Applied Biosystems, Foster City, California, USA).
pERK 1/2 immunohistochemistry
Rats were deeply anaesthetised with sodium pentobarbital (Nembutal 70 mg/kg intraperitoneally; Abbott) and perfused through a cardiac-aorta cannula with 500 ml/rat of 4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer solution (pH 7.2). The lumbosacral spinal cord (L6–S1) was dissected out and post-fixed in the same fixative overnight and cryoprotected by immersion in 10% sucrose for one day. The L6–S1 segment was transversally sectioned at 30 μm with a cryostat. Free floating sections were processed with ABC techniques with slight modifications. Sections were incubated with rabbit anti-pERK (Phospho-pERK 44/42 Map Kinase antibody from Cell Signaling Technology, Inc., Beverly, Massachusetts, USA) at 1:2000 dilution for two nights at 4°C, in biotinylated secondary goat antirabbit IgG at 1:1000 for 1.5 hours, and lastly with avidin-biotin peroxidase complex at 1:400 for one hour at room temperature. The chromogen was 3,3-diaminobenzedine tetrahydrochloride deposited as dark brown labelling in the presence of H2O2. The number of pERK 1/2 immunoreactive cells in laminae I and IIo was counted unilaterally in 15 sections randomly selected and expressed as cells/unilateral section. All samples were handled under the same experimental conditions.
Colonic tissue damage assessment after in vivo CRD
Distal colonic tissues were visually observed for macroscopic damage and scored based on colonic wall thickness and the presence of lesions such as ulcerations, as described previously.29 For histological analysis, tissues were fixed by overnight immersion in neutral buffered formalin (4%), embedded in paraffin wax, sectioned (6 µm), stained with haematoxylin and eosin, and examined by light microscopy. Crypt architecture, vascular and colonic wall damage, and the presence of inflammatory cells were assessed. In rare cases (<5%), when there were signs of colonic damage caused by balloon placement, as evidenced by the presence of blood on the balloon, data were disregarded.
Experimental protocols
Effect of intravenous hUcn 2 on VMR to two CRDs in conscious rats
In non-fasted rats, chronically implanted with abdominal electrodes, 60 minutes after balloon and intravenous catheter placement, a 10 minute basal EMG recording was made and then the CRD protocol (60 mm Hg for 10 minutes twice, with a 10 minute rest interval) was initiated. In the first set of experiments, hUcn 2 (10 or 20 μg/kg) or saline was injected intravenously at the end of the first distension. In a second set, astressin2-B (200 μg/kg) or its vehicle (distilled water) was injected subcutaneously (0.5 ml) 20 minutes before the onset of the first distension, and hUcn 2 (20 μg/kg) or saline (0.1 ml) was injected intravenously at the end of the first distension. In another set of experiments, saline or hUcn 2 was given intravenously (20 μg/kg) 20 minutes before the beginning of the distension protocol. The VMR to the second distension was compared with that of the first distension response. Per cent changes between the two CRDs and the different treatment groups were compared. Doses of peptides and routes of administration were based on our previous studies, where under similar conditions hUcn 2 exerted biological actions on upper gut motor function that were blocked by astressin2-B.30
In separate studies, three groups of rats, naïve (no treatment), sham (balloon inserted into the colorectum without CRD), and CRD (60 mm Hg twice, with a 10 minute rest interval) were euthanised at the time corresponding to the end of the two CRDs. Colons were processed for macroscopic and histological examinations.
Effect of peripheral Ucn 2 on locomotor activity
Rats were injected intraperitoneally with saline or hUcn 2 (20 μg/kg) and 10 minutes later placed in an open field white box (50 cm×50 cm×22 cm) with the floor divided into nine squares. The number of crossed squares (locomotor activity score) was recorded for every 10 minutes for 30 minutes. Between each trial, the floor was cleaned with alcohol to avoid scent.
Effect of peripheral Ucn 2 on distal colonic transit in conscious rats
Rats fasted for 24 hours had free access to a preweighed chow for a two hour period and then a plastic bead (5 mm diameter) was inserted into the distal colon (4 cm proximal to the anus) as in previous studies,30 and saline or hUcn 2 (20 μg/kg) was injected intravenously through the tail vein. The time required to expel the bead (minutes) was monitored over four hours.
Effect of CRD and intravenous hUcn 2 on lumbosacral neuronal ERK 1/2 activation and VMR
Sixty minutes after balloon and intravenous catheter placements, rats were injected intravenously with either saline (0.1 ml) or hUcn 2 (20 μg/kg), and 10 minutes later a single 10 minute CRD (60 mm Hg) or no distension (sham) was applied. Abdominal contractions were identified visually as a snaky contraction of the abdomen with inward turning of the hind limb, hump backed position, or squashing of the lower abdomen against the floor, as previously described.31 Counting of contractions was done for 10 minutes after intravenous treatment and during the 10 minutes of CRD or no CRD. Such visual VMR assessment avoids the possible confounding effect of chronic surgery and electrodes on spinal neuronal activation monitored in the study. At the end of CRD, all groups were perfused under deep anaesthesia, euthanised, and the lumbosacral spinal cord (L6–S1) processed for pERK 1/2 immunohistochemistry.
In vitro colorectal preparation
Each experiment included a 30 minute stabilisation period followed by five minutes of initial basal ISA recording. At the end of each experiment, bradykinin (1 μg/0.1 ml = 4.9×10−6 M) was injected intra-arterially to assess afferent nerve fibre responsiveness. This was followed, 10 minutes later, by search of the RF and injection of toluidine blue (0.02% intra-arterially) to verify arterial patency. All intra-arterial injections were performed in 0.1 ml over 20 seconds.
In the first study, the ISA response to graded intensities of CRD was achieved by inflating the balloon with increasing volumes of air (0.1, 0.2, 0.3, 0.4, and 0.5 ml). Each CRD was maintained for 20 seconds and occurred within a ∼5–10 minute interval. Based on the results, 0.4 ml CRD for 20 seconds was used as a standard distension protocol. In the second study, intra-arterial vehicle was injected two minutes before CRD and this paradigm was repeated four times at ∼6 minute intervals. In the third study, sequential treatments were performed: vehicle intra-arterially, CRD, hUcn 2 (1 μg/0.1 ml = 1.87×10−6 M, intra-arterially), CRD, hUcn 2 (2 μg/0.1 ml = 3.75×10−6 M intra-arterially), CRD, Ucn 3 (3 μg/0.1 ml = 5.62×10−6 M, intra-arterially), CRD, astressin2-B (20 μg/0.1 ml = 4.94×10−5 M, intra-arterially), Ucn 2 (3 μg intra-arterially), and CRD. There was a two minute interval between intra-arterial injection and CRD, and a 10 minute between CRD and the next intra-arterial injection.
Data analysis
All values, unless otherwise indicated, are mean (SEM). Repeated measures paired t test was used to compare the first with the second AUC, and the first versus the second number of abdominal contractions as well as for in vitro ISA spike activity before and after injection or before and during CRD. AUC per cent differences between the two CRD responses among treatment groups, distal colonic bead expulsion time, and in vitro ISA activity comparison between groups was analysed using one way ANOVA followed by the Newman-Keuls multiple comparison test. A p value of <0.05 was considered statistically significant.
RESULTS
Ucn 2 injected intravenously decreases repeated CRD induced VMR in conscious rats chronically implanted with intramuscular abdominal electrodes
In rats injected intravenously with saline just after the first CRD, there was a 30.6 (4.0)% (mean (SEM)) increase (p<0.05) in AUC during the second CRD compared with its first response (figs 1A, B; 2A), indicating sensitisation. hUcn 2 (10 and 20 μg/kg), injected intravenously after the first CRD, prevented the increase in AUC to the second CRD (figs 1B, 2A). In addition, at 20 μg/kg, hUcn 2 decreased the AUC response from 33.1 (4.5) V×s (first response) to 23.0 (3.0) V×s (second response; p<0.05 v first response) resulting in a 30.5 (4.8)% reduction in visceral pain response proper (fig 2A). Prior administration of the CRF2 antagonist, astressin2-B (200 μg/kg subcutaneously) blocked the inhibitory effect of hUcn 2 (20 μg/kg) on the second response compared with the first response (fig 2B). Astressin2-B (200 μg/kg subcutaneously) alone did not influence the sensitisation response (fig 2B).
Representative illustration of abdominal contraction traces (A) and individual AUC responses to the first and second colorectal distension (CRD) in saline and human urocortin 2 (hUcn 2) treated rats (B). Rats were chronically implanted with abdominal electrodes and ∼10 days later were briefly anaesthetised with isoflurane and fitted with an intracolonic balloon. After a 60 minute recovery period and habituation to Bollmann cages, they were subjected to two 10 minute CRDs at 60 mm Hg with a 10 minute rest interval. Saline or hUcn 2 was injected intravenously (0.25 ml) just at the end of the first CRD. Note that the abdominal contraction response to the same distension during the second set is higher than the first set. Values in (A) are electromyogram (EMG) voltage whereas in (B) values are area under the curve (AUC) units of the response to the first and second CRD.
Effect of human urocortin 2 (hUcn 2) (A) and astressin2-B (B) on tonic repeated colorectal distension (CRD) induced visceral pain/hypersensitivity in conscious rats. Rats were chronically implanted with abdominal electrodes and ∼10 days later were injected subcutaneously (sc) with vehicle (water) or astressin2-B. After 10 minutes, rats were subjected to the first CRD (10 minutes at 60 mm Hg) followed by intravenous (iv) injection of saline or hUcn 2. Rats were allowed to rest for 10 minutes and received the second distension (60 mm Hg, 10 minutes). Values are mean (SEM) of total per cent differences between the first and second responses to CRDs. *p<0.05 versus saline or hUcn 2 (20 μg/kg); †p<0.05 versus all other groups (n = 5–6 rats/group, ANOVA).
When hUcn 2 (20 μg/kg intravenously) was given 20 minutes before the first CRD distension, the responses to the two CRD distensions were similar (20.6 (3.3) v 23.5 (4.6) V×s; p>0.05, n = 6) whereas after intravenous vehicle the second response was significantly higher than the first (29.8 (4.2) v 38.3 (6.4) V×s; p<0.05, n = 6).
Tonic repeated CRD did not cause colonic tissue damage in rats
Macroscopic score for the colons of naïve, sham, and CRD (60 mm Hg for 10 minutes twice, with a 10 minute rest interval) groups were not different (1.8 (0.3), 2.0 (0.2), and 2.2 (0.4), respectively; p>0.05). Similarly, histological analysis did not reveal differences in crypt and colonic wall structure or presence of inflammatory cells (fig 3). However, in two of the four CRD rats, the submucosal space was enlarged compared with the other rats.
Photomicrographs of distal colon tissues in the naïve (A = no balloon placement and no distension), sham (B = balloon placed into the colorectum but no colorectal distension (CRD)), and distension (C = rats fitted with balloon and subjected to two 10 minute, 60 mm Hg CRD) groups. Colonic tissues were processed for haematoxylin and eosin staining at the end of the second CRD. Magnification ×100. Note that there are no architectural or inflammatory response differences between the different groups except the enlarged submucosal space in the CRD group.
hUcn 2 injected intraperitoneally did not alter locomotor activity in naïve rats
Rats injected with intraperitoneal saline (n = 7) crossed 65.3 (7.9), 11.7 (2.5), and 9.1 (2.2) squares during the first, second, and third 10 minute periods of observation, respectively. hUcn 2 (20 μg/kg intraperitoneally, n = 7) did not affect (p>0.05) the locomotor activity score at any of the time points (squares crossed/10 min: 47.7 (7.3), 16.4 (4.9), and 14.7 (3.1), respectively). Similarly, the number of squares crossed during the total 30 minute period was not different between saline and hUcn 2 (20 μg/kg) injected rats (saline 86.1 (10.4) v hUcn 2 78.8 (10.1); p>0.05).
hUcn 2 injected intravenously did not alter distal colonic transit in naïve rats
In fasted rats (n = 6), distal colonic transit, assessed by bead expulsion time, was 155.6 (27.4) minutes. Neither intravenous saline nor hUcn 2 (20 μg/kg) altered distal colonic transit time (148.0 (32.2) minutes (n = 10) and 169.5 (33.4) minutes (n = 8), respectively).
CRF2 receptor gene expression
CRF2 receptor expression was detected by RT-PCR in the heart and T13–S1 DRGs, and spinal cord tissues in naïve rats (fig 4). Primers were designed to target the core regions of the rat CRF2 gene in the area corresponding to the transmembrane domains and cytoplasmic tail of the receptor protein common to functional splice variants, CRF2a and CRF2b.
Corticotropin releasing factor 2 (CRF2) gene expression in rat dorsal root ganglia (DRG) and spinal cord, at T13–S1 segments, detected by reverse transcriptase-polymerase chain reaction (RT-PCR). Images are from one rat representative of 3–6 rats studied. Each band of the DRG RT-PCR products was pooled from the ganglia of both left and right sides. Lanes 1–7: DRG T13–L4, L6, S1. Lanes 8–9: spinal cord T13–L1, L3–4, L6–S1. ARP, acetic ribosomal protein (housekeeping gene); Ht, heart (CRF2 gene expression control); Cont, negative control (without RT).
CRD induced spinal ERK 1/2 phosphorylation and abdominal contractions were attenuated by intravenous hUcn 2 in conscious rats
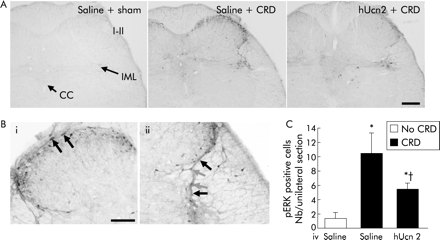
A single CRD (60 mm Hg), induced robust activation of ERK1/2 bilaterally in the lumbosacral spinal cord, as monitored at the end of the 10 minute CRD in the intravenous saline treated group. pERK1/2 immunoreactivity was observed in the superficial dorsal horn (laminae I and IIo), along the lateral collateral visceral afferent pathway and the intermediolateral column (IML; fig 5A, B). A few pERK positive cells were observed in the non-distended sham group (fig 5A,C). CRD, under these conditions, induced abdominal contractions (number/10 min: 42.3 (11.5) v sham 2.3 (0.8); p<0.05). hUcn 2 (20 μg/kg intravenously 10 minutes before CRD) blunted the phosphorylation of ERK1/2 in the above spinal regions (fig 5A,C). The number of pERK1/2 positive cells in the laminae I and IIo induced by CRD was significantly reduced from 10.4 (2.9) in saline to 5.4 (1.0)/unilateral section after intravenous hUcn 2 (fig 5C). hUcn 2 (20 μg/kg intravenously) also decreased the number of contractions/10 minutes to 21.0 (3.2) versus saline 42.3 (11.5) (p<0.05).
Modulation of extracellular signal regulated kinase (ERK) 1/2 activation in the spinal cord L6–S1 by human urocortin 2 (hUcn 2) in rats. (A) Photomicrograph of phosphorylated ERK (pERK) 1/2 immunoreactivity. Under brief isoflurane anaesthesia, rats were fitted with a rectocolonic distension balloon and tail vein catheter and kept in Bollmann cages for 60 minutes. Conscious rats were then injected intravenous with saline or hUcn 2 (20 μg/kg) and 10 minutes later received either a single 10 minute colorectal distension (CRD) at 60 mm Hg or no distension (sham). At the end of the distension period, rats were killed for pERK 1/2 immunohistochemistry. I–II, laminae I–II; IML, intermediolateral column; CC, central canal. Scale bar 100 μm. (B) Higher magnifications from saline + CRD group showing the presence of pERK 1/2 in the larger neurones of laminae I and smaller neurones of the outer laminae II (i), as well as in neurones and fibres (arrows) along the lateral collateral visceral afferent fibres (ii). Scale bar 100 μm. (C) Mean (SEM) number of pERK 1/2 positive cells in the laminae I and IIo of the lumbosacral spinal cord of rats. *p<0.05 versus all other groups; †p<0.05 versus saline + CRD group (n = 4 rats/group, ANOVA). Note that in this experiment hUcn 2 or saline was given intravenously (iv) prior to the first distension.
In rats injected with intravenous saline, the first and second CRD induced 30.5 (6.6) and 43.2 (5.8) contractions/10 minutes, respectively (p<0.05; a 42% increase in response to the second distension). In rats injected intravenously with hUcn 2 (20 μg/kg) before any distension, both the first and second responses were significantly lowered compared with intravenous saline (21.2 (3.5) v 30.5 (6.6) contractions/10 minutes for the first VMR and 22.3 (3.6) v 43.2 (5.8) contractions/10 minutes for the second VMR; p<0.05).
Ucn 2 injected intra-arterially reduces CRD induced ISA fibre activity in vitro
Incremental balloon distensions at ∼6 minute intervals induced volume dependent increases in ISA activity (fig 6A, B). In 23 units from 11 in vitro rat preparations, basal impulse/20 seconds was significantly increased from pre-distension values of 17 (4) to 47 (10) at 0.3 ml, 16 (3) to 67 (12) at 0.4 ml, and 21 (4) to 94 (16) at 0.5 ml distension volumes, while CRD at 0.1 ml had no effect and the increase observed at 0.2 ml did not reach significance (basal 15 (4) to 29 (8)) (fig 6B). Fifteen minutes after the last CRD, the 23 units responsive to CRD were also responsive to intra-arterial injection of bradykinin (1 μg/0.1 ml), suggesting that the units were multimodal that responded to physical and chemical stimuli. Bradykinin resulted in a significant increase in basal ISA activity from 103 (25) to 465 (68) spikes/100 seconds while intra-arterial injection of vehicle had no effect (pre injection 69 (13); vehicle 77 (14) spikes/100 seconds; 23 units analysed from the same 11 preparations). Based on these data, CRD at 0.4 ml for 20 seconds was selected for further study. Repeated vehicle intra-arterial injections and CRD (four times at ∼6 minute intervals) induced a similar increase in ISA impulses/20 seconds, as shown in six single units analysed from three experiments (fig 6C).
Effects of repeated colorectal distensions (CRDs) on inferior splanchnic afferent (ISA) spike activity in an in vitro colorectal preparation from fasted rats. (A) Representative spike counts versus time histogram showing the response of one unit to graded volume CRD (0.1–0.5 ml, 20 seconds each). Bin width is 14 seconds. The top two insets in (A) show, in a faster time scale, the stimulus-response function for 0.1 and 0.4 ml CRDs. (B) Twenty three ISA fibre units analysed from 11 rat in vitro preparations (1–4 units per preparation). CRD (0.3–0.5 ml) induced a volume dependent significant increase in ISA. (C) Similar ISA response to repeated CRDs (0.4 ml, 20 seconds each). Vehicle was injected intra-arterially two minutes before each CRD. Values for (B) and (C) are mean (SEM) of 20 second spike counts pre and during CRD in six ISA fibre units analysed from three rat in vitro preparations (1–3 units/preparation). *p<0.05, ***p<0.001 versus pre-distension.
Intra-arterial injections of vehicle, hUcn 2 at 1, 2, and 3 μg, and astressin2-B (20 μg) per se did not alter basal ISA activity (138 (23)/147 (29), 166 (33)/206 (50), 147 (30)/180 (31), 142 (28)/195 (27), and 125 (28)/142 (32) per 100 seconds, respectively; n = 8 single units analysed from four experiments). In the same preparations, consecutive intra-arterial injections of Ucn 2 (1, 2, or 3 μg) two minutes before CRD dose dependently lowered the CRD induced ISA response to 62 (14), 54 (14), and 44 (10) spikes/20 seconds, respectively, compared with intra-arterial vehicle + CRD (78 (15) spikes/20 seconds), with significant inhibition at 3 μg hUcn 2 (fig 7B). Astressin2-B, which did not influence the CRD response in itself, prevented the Ucn 2 (3 μg) inhibitory effect (fig 7A,B). Fifteen minutes after the last CRD, intra-arterial injection of bradykinin (1 μg) resulted in a significant increase in basal impulse activity of ISA to 544 (91) spikes/100 seconds (p<0.05 v pre-injection 119 (35)) (same eight units from four preparations) (fig 7B).
Effects of close intra-arterial (inferior mesenteric artery) injection of human urocortin 2 (hUcn 2) on colorectal distension (CRD) induced inferior splanchnic afferent (ISA) spike activity in an isolated colorectal preparation from fasted rats. (A) Representative single unit response to CRD (0.4 ml for 20 seconds) after vehicle (saline 0.1 ml intra-arterially), hUcn 2 (3 μg, 0.1 ml), or astressin2-B (20 μg, 0.1 ml plus hUcn 2, 3 μg, 0.1 ml). F, flush; ia, intra-arterial. The lower panels show the raw data, at a faster time scale, for the single unit response in the astressin2-B + hUcn2 trace (left panel, time starts at 111 seconds; right panel time starts on the first second of CRD). Raw data were output using Spike 2 (CED Ltd, Cambridge, UK). (B) hUcn 2 dose dependently inhibited the distension induced rise in ISA spike frequency. The inhibitory effect of hUcn 2 was prevented by astressin2-B (Ast2-B). *p<0.05 versus pre-CRD; †p<0.05 versus vehicle (Ve) + CRD. (C) Bradykinin (BK 1 μg, 0.1 ml) injected at the end of the experiment caused robust ISA fibre activity. *p<0.05 versus pre-injection of BK. Values are mean (SEM) of 20 seconds pre and during CRD in the hUcn 2 study or 100 seconds pre and after bradykinin injection in eight single units from four rat in vitro preparations (1–3 units/preparation).
DISCUSSION
The present study shows that two repeated tonic noxious CRDs in awake rats produce visceral sensitisation and phosphorylation of ERK 1/2 in laminae I and IIo of the lumbosacral spinal cord known to receive visceral afferent input from the pelvic organs.20 Activation of peripheral CRF2 receptors by intravenous hUcn 24 dampened the tonic CRD induced visceral pain and spinal ERK1/2 phosphorylation, and blocked the sensitisation without affecting locomotor activity or distal colon transit. Injection of hUcn 2 also blunted CRD induced ISA activation in an in vitro isolated colorectal preparation. The in vivo and in vitro actions of hUcn 2 were prevented by the CRF2 receptor antagonist, astressin2-B, that by itself did not influence visceromotor or ISA responses to CRD. CRF2 receptor gene expression was detected by RT-PCR in the DRG and spinal cord within the T13–S1 segments from naïve rats. These findings provide evidence that intravenous Ucn 2-induced CRF2 receptor mediated blunting of visceral pain and inhibition of sensitisation to repeated CRD may involve modulation of colonic afferent fibre activity and ERK1/2 phosphorylation in spinal neurones receiving visceral input.
Development of visceral sensitisation by two repeated tonic CRDs
IBS patients subjected to successive incremental distensions of the rectum displayed a lowered rectal pain threshold compared with normal subjects.32 Other studies showed that rectal hyperalgesia to repeated CRD was specific to IBS.33 Several experimental models inducing visceral hyperalgesia used intracolonic application of chemical irritants,24 exposure to acute or chronic stress,34–36 or early life visceral pain in rats.37 Our data provide evidence that two 10 minute on/off noxious CRDs (60 mm Hg) induced visceral pain/sensitisation in awake rats, either naïve or fitted with chronic abdominal electrodes. Similarly, other CRD models involving 10 repetitive phasic CRDs (30 or 50 mm Hg, 60 seconds on/30 seconds off)38 or two 10 minute CRDs (60 mm Hg) separated by 10 phasic CRDs (80 mm Hg, 15 seconds on/30 seconds off)39 resulted in visceral sensitisation in awake rats. It has been reported that repeated CRD (80 mm Hg, 60 times, 30 seconds on/90 seconds off for two hours) induced signs of inflammation.20 However, under our conditions, except for an enlarged submucosal space, the colonic wall, examined macroscopically and histologically, revealed no sign of inflammation or damage at the end of the two 10 minute CRDs applied with a 10 minute rest interval. As enhanced colonic mechanosensation in the absence of colonic injury is the hallmark of IBS,32,33 the present CRD paradigm, which does not produce tissue damage, is a relevant experimental model to study modulation of visceral sensitisation.
hUcn 2 inhibition of CRD induced visceral pain/sensitisation and splanchnic afferent activity
Activation of CRF2 receptors blunted visceral pain and prevented sensitisation induced by noxious CRD. This is supported firstly by the demonstration that the selective CRF2 agonist, hUcn 2,4 injected intravenously, reduced visceral pain and inhibited the sensitisation to CRD in awake rats. Secondly, the effects of intravenous hUcn 2 were completely blocked by the selective CRF2 antagonist, astressin2-B.7 In contrast, we previously reported that activation of CRF1 receptor using the preferential CRF1 ligand, ovine CRF,5 lowered the sensation of discomfort to the colon in normal human subjects.10 It is unlikely that inhibition of VMR to intravenous hUcn 2 may have been confounded by behaviour changes as we showed that peripheral injection of hUcn 2 did not affect the locomotor activity response to an open field environment. The analgesic effect of hUcn 2 to CRD was also not secondary to alterations in colonic motility because hUcn 2, injected at a dose modulating the visceral pain response to CRD, did not affect basal propulsive motor function of the distal colon, as reported previously.30 The absence of basal modulation of distal colonic transit by peripheral activation of CRF2 receptors contrasts with the potent stimulation of colonic motility by activation of peripheral CRF1 receptors in rats and mice.9 Taken together these observations are consistent with CRF2, unlike CRF1, receptors mediating intravenous Ucn 2 inhibitory effects on pain/sensitisation and provide the first evidence of visceral pain modulation by CRF2 receptor activation.
Sensory information from the distal colon/rectum is conveyed through the inferior (lumbar) splanchnic nerves which project to the thoracolumbar spinal cord and through pelvic nerves that enter the lumbosacral cord.40 In vivo and in vitro electrophysiological studies in rodents have shown that CRD activates mechanosensitive afferents located in both splanchnic and pelvic fibres.41–43 Although CRF2 receptors have been detected within the rat and human colon,44,45 there is evidence that peripherally administered Ucn 2 can also reach the brain parenchyma at a moderate rate.46 Therefore, we used a novel in vitro approach to assess whether Ucn 2 acts peripherally to modulate CRD induced activation of ISA activity. In an isolated rat colorectal ISA-inferior mesenteric artery preparation, phasic CRD increased ISA impulses, and this response was dose dependently reduced by intra-arterial injection of hUcn 2. In addition, hUcn 2 inhibitory action was blocked by intra-arterial astressin2-B, which by itself did not influence significantly the ISA response to CRD. The intensity encoding properties of ISAs was demonstrated by the linear increase in their activity with 0.1 ml ascending volumes of balloon inflation from 0.1 to 0.5 ml and the reproducible ISA firing activity in response to repeated CRD of similar intensity (0.4 ml). In addition, the single unit responsive to phasic CRD had a robust response to intra-arterial injection of bradykinin, which consistently excites visceral nociceptors.41 Similar patterns were reported in rat pelvic afferent fibres in response to graded or similar phasic CRD and bradykinin injection in vivo.41 These data indicate that intravenous hUcn 2 induced dampening of visceral pain response to noxious CRD may occur through peripheral activation of CRF2 receptors that blunts ISA activation to CRD. Although the lack of sensitisation of in vitro ISA fibre activity in response to repeated distensions argues for central sensitisation as a major mechanism for the observed visceral hyperalgesia in awake rats, the in vitro ISA fibre activity study was done using short phasic (20 seconds) distension and intensity (0.4 ml). These may not induce the necessary temporal and intensity components to cause increased excitability of ISA fibres.
hUcn 2 modulates ERK 1/2 activation in lumbosacral dorsal horn by CRD
A number of recent studies have demonstrated that activation of Aδ or C fibres by noxious thermal, mechanical, chemical, or electrical stimuli of somatic origin induce rapid activation (phosphorylation) of two mitogen activated protein kinases (MAP kinases), p42 and p44, referred to as ERK 1 and 2 (ERK 1/2), in lamina I and IIo neurones of the spinal cord, and that ERK 1/2 activation is involved in the pain response.18 Colorectal afferents projecting to the spinal cord are mostly composed of C (unmyelinated) or Aδ (small myelinated) fibres.40 While much attention has been focused on ERK signal transduction mechanisms responsible for the modulation of somatic pain/hyperalgesia,18,47,48 little is known as to whether such pathways are part of the visceral pain/sensitisation process. One study in awake mice indicated that intracolonic instillation of capsaicin or mustard oil resulted in a rapid increase in pERK 1/2 selectively in lumbosacral spinal cord, as measured by western blot analysis.19 In the present study, we showed that noxious CRD applied for 10 minutes in naïve rats induced phosphorylation of ERK 1/2 in lumbosacral spinal cord neurones. pERK labelling was observed within 10 minutes and topographically located in discrete areas of the dorsal horn (lamina I, IIo, and lateral collateral visceral afferent nerve bundles and IML). These data indicate that visceral pain induced by noxious CRD can rapidly induce activation of the MAPK (ERK) pathways in individual neurones of the spinal cord at laminar locations where primary afferent C fibres terminate.20,40 Therefore, examination of the presence of phosphorylated ERK 1/2 in the superficial dorsal horn may be a useful marker of postsynaptic neurones actively responsive to visceral pain.
Several studies also established that rapid ERK phosphorylation plays an important role in hypersensitivity after noxious somatic stimuli and inflammation by modulating neuronal excitability.49 In a parallel study to ERK 1/2 activation, rats subjected to a second CRD displayed 42% enhanced abdominal contraction, suggesting similar sensitisation occurring in naïve rats in the pERK 1/2 study compared with those fitted with chronic abdominal electrodes. Peripheral administration of hUcn 2 blunted CRD induced ERK 1/2 activation, as well as VMR, indicating a correlation between the VMR response and pERK 1/2 immunostaining occurring in spinal cord laminae I and IIo. Although yet to be confirmed, these data suggest that the inhibitory action of hUcn 2 against repeated CRD induced visceral sensitisation may involve reduction of spinal ERK 1/2 activation. Given the rapid time frame of hypersensitivity induced by CRD (<1 hour), ERK activation may mainly induce post-translational changes in ion channels or receptors modulating neuronal excitability.49 hUcn 2 may modulate pERK 1/2 induction through inhibition of CRD induced primary afferent activation that conveys nociceptive messages to the spinal cord. Gene expression of CRF2 receptors in the lumbosacral DRG of rats demonstrated by RT-PCR, supports the involvement of CRF2 in hUcn 2 action and the possible post-translational change induced by ERK 1/2 activation. However, of note is that the afferents innervating the colon constitute the minority of DRG cells that were harvested. In addition, systemic injection of Ucn 2 has been reported to have some access to brain parenchyma,46 and we showed expression of CRF2 receptors in the spinal cord by RT-PCR. Whether intravenous hUcn 2 has access to the spinal cord to act directly on spinal neurones leading to reduced ERK 1/2 phosphorylation needs to be investigated further.
This study presents another paradigm, visceral pain/sensitisation, in which CRF2 receptor activation exerts an opposite effect to that of the CRF1 receptor.50,51 These data lend support to the concept that susceptibility to stress or noxious stimuli may be viewed not only in the context of exaggerated activation of CRF1 pathways but also from an inadequate mounting of CRF2 signalling mechanisms that curtail the CRF1 mediated stress response. The present demonstration of the analgesic effect of CRF2 receptor activation in a model of visceral pain/sensitisation may also be relevant to the increased interest in the potential use of CRF receptor modulation in the treatment of IBS.9,13,52
Acknowledgments
This work was supported by NIH grants, R01 DK-33061 (YT), RO1 DK-57238 (YT), DK41301 (Animal Model Core, YT, MM), DK-57238-01A1S1 (MM), P-50 DK64539 (EAM, YT, MM), and DK26741 (WV, JR). We thank AstraZeneca, Timothy Maryanov, Marciano Sablad, JingFang Zhao, and Honghui Liang for the material and technical support provided. We would like also to thank Teresa Olivas for editing the manuscript.
REFERENCES
Footnotes
-
Published online first 28 June 2005
-
Conflict of interest: None declared.