Frontal asymmetry assessed in infancy using functional near-infrared spectroscopy is associated with emotional and behavioral problems in early childhood
Michelle Bosquet Enlow and Charles A. Nelson co-senior authors.
Abstract
Frontal asymmetry (FA), the difference in brain activity between the left versus right frontal areas, is thought to reflect approach versus avoidance motivation. This study (2012–2021) used functional near-infrared spectroscopy to investigate if infant (Mage = 7.63 months; N = 90; n = 48 male; n = 75 White) FA in the dorsolateral prefrontal cortex relates to psychopathology in later childhood (Mage = 62.05 months). Greater right FA to happy faces was associated with increased internalizing (η2 = .09) and externalizing (η2 = .06) problems at age 5 years. Greater right FA to both happy and fearful faces was associated with an increased likelihood of a lifetime anxiety diagnosis (R2 > .13). FA may be an informative and early-emerging marker for psychopathology.
Abbreviations
-
- ADHD
-
- attention-deficit/hyperactivity disorder
-
- CBCL
-
- Child Behavior Checklist
-
- deoxyHb
-
- deoxygenated hemoglobin
-
- DIPA
-
- Diagnostic Infant Preschool Assessment
-
- dlPFC
-
- dorsolateral prefrontal cortex
-
- DSM
-
- Diagnostic and Statistical Manual of Mental Disorders
-
- EEG
-
- electroencephalography
-
- FA
-
- frontal asymmetry
-
- fNIRS
-
- functional near-infrared spectroscopy
-
- OD
-
- optical density
-
- ODD
-
- oppositional defiant disorder
-
- oxyHb
-
- oxygenated hemoglobin
Beginning in the first weeks of life, infants are continuously exposed to emotional stimuli in the form of faces and voices. However, not all infants respond the same way to the same stimuli, and these individual differences may be relevant to the development of psychopathology in childhood. Aberrant (i.e., heightened and attenuated) responses to emotional situations are a common phenotype exhibited in both internalizing and externalizing disorders, suggesting developmental and possibly shared neural origins (Rubin et al., 2009). The overall objective of the present study was to use near-infrared spectroscopy (fNIRS) to investigate if and how infant frontal brain asymmetry (FA) responses, or the relative difference in brain activity between left versus right frontal areas, to emotional stimuli are associated with later childhood emotional and behavioral outcomes.
Frontal alpha asymmetry assessed using electroencephalography (EEG) is a well-studied biological correlate of motivation behaviors in infants, children, and adults (Davidson, 1995). Measurements in the alpha band frequency using EEG are associated with a reduction in brain activity. Specifically, greater relative left FA (or decreased relative left alpha power) reflects an approach-oriented response (e.g., curiosity, surgency), whereas greater relative right FA (or decreased relative right alpha power) reflects a withdrawal-oriented response (e.g., fear; Davidson, 1995; Harmon-Jones & Allen, 1998). FA is found to be relatively stable across infant development (Brooker et al., 2017). Although the existing developmental work has examined FA and concurrent associations with temperament during infancy and through the first 4 years of life, it has failed to examine how infant FA may be related to later outcomes in early childhood (Fox et al., 2001; Smith & Bell, 2010). This gap in the literature is especially important to address, as early childhood is an important developmental period for observing the first signs and onset of anxiety and other behavior disorders (Cartwright-Hatton et al., 2006; Headley & Campbell, 2011; Kovacs & Devlin, 1998).
Notably, greater relative right EEG FA in infancy has been considered a risk marker for maladaptive stress responding (Buss et al., 2003; Davidson, 2004) and several psychological disorders, including depression (Thibodeau et al., 2006) and anxiety (Davidson et al., 2000). However, the literature is inconsistent, with some studies failing to find an association between right FA and depression (Kołodziej et al., 2021; Reid et al., 1998). The pattern is even more inconsistent for externalizing disorders, with some finding no effect and some finding that greater left FA (indexing approach motivation) is associated with externalizing problems (Peltola et al., 2014; Smith & Bell, 2010). Thus, more research is needed to better specify the conditions under which FA is reliably associated with later psychological problems.
The noted inconsistencies across studies may be related to the affective context of the measurement session. To date, most EEG studies linking FA to psychopathology used a resting or non-social context, thought to capture a stable temperamental trait (Coan & Allen, 2003). However, studying asymmetry within a particular context has the potential to clarify previous associations because it minimizes uncontrolled variance (e.g., mind-wandering, current affective state) introduced in a resting-state session. Additionally, some posit FA is a marker of adaptability and regulatory ability to a particular environmental context or stressor (capability model; Coan et al., 2006). Similarly, some EEG studies have argued FA is more appropriate as a moderator or a mediator rather than a predictor of emotion regulation. In this context, the capability model and the present study treat FA as the moderator, and affective response as the predictor (Coan et al., 2006; Reznik & Allen, 2018). To this point, adults with depression and anxiety had increased relative right EEG FA to both positive and negative emotional contexts (e.g., public speaking task and making positive and negative emotional faces) compared with neurotypical controls (Davidson et al., 2000; Stewart, Coan, et al., 2011). Moreover, greater relative right FA to a negative emotional context (i.e., threat of shock), but not at rest, has been associated with a heightened stress response (assessed using startle; Goodman et al., 2013). Overall, the adult work suggests that withdrawal (e.g., greater relative right FA) from both positive and negative emotional contexts may be associated with a greater risk for internalizing symptoms, and more work is needed to better understand the relation between FA response to emotional stimuli and externalizing symptoms.
Furthermore, little is known about how individual differences in FA response to an emotional challenge during infancy is associated with later emotional and behavioral problems (Davidson & Fox, 1982). This is important, as early experiences shape the development of long-term response patterns. For example, infants who are predisposed to respond to emotional input from a caregiver by withdrawing due to underlying greater relative right FA may elicit a change in engagement efforts from their caregiver (e.g., increased withdrawal or increased approach). Both types of caregiver responses, increased approach and increased withdrawal, may reinforce withdrawal behaviors from the child (Fisak & Grills-Taquechel, 2007).
To date, there has been little work in either adult or pediatric populations that has compared how the brain–behavior associations for FA change across affective contexts (i.e., FA responses to positive vs. negative emotions), which would help clarify if generalized response patterns (e.g., withdrawal to happy, angry, and fearful faces) or context-specific response patterns (e.g., withdrawal to happy faces and approach to fearful faces, indexing a mismatch to the environment) are linked to psychopathology risk. Preliminary evidence in early childhood has shown concurrent associations, such that FA responses assessed using EEG to happy, but not to neutral or sad films, are associated with depressive symptoms (Feng et al., 2012). Assessing FA in in response to emotional faces during infancy is especially important given that differential behavioral and prefrontal cortex responses to emotional faces emerge at this time (for a review see Ruba & Repacholi, 2020). Prior work has found that individual differences in both positive and negative emotion facial recognition assessed using EEG, eye tracking, and behavioral assessments are linked to later developmental outcomes (Herba & Phillips, 2004; Luyster et al., 2014; Pollak & Sinha, 2002). Thus, more developmental work is needed to understand if FA patterns in infancy can provide informative markers for later psychological outcomes (Peltola et al., 2014; Smith & Bell, 2010).
In addition, it is possible that mixed findings between EEG FA and psychopathology are due in some part to the limitations of EEG methodology (see Kołodziej et al., 2021). Therefore, we may gain further insights into FA by leveraging other imaging modalities, such as fNIRS. Unlike EEG which measures neuronal activity, fNIRS measures metabolic changes in the cortex through the assessment of oxygenated and deoxygenated hemoglobin (oxyHb and deoxyHb). Moreover, fNIRS is a non-invasive, non-restrictive, and safe optical neuroimaging technique well-suited for use with pediatric populations. Preliminary work with fNIRS has shown that FA patterns differ across ratings of infant behavioral temperament (high vs. low ratings of negative emotionality; Ravicz et al., 2015) and genetic risk (risk allele vs. no risk allele; Krol et al., 2021) but is not associated with maternal depression or anxiety (Porto et al., 2020). Given the differences in the modalities and limited work, which has assessed FA with fNIRS it is unknown how much overlap there is between FA assessed using EEG and fNIRS. Compared with EEG, fNIRS offers superior spatial resolution, allowing more precise localization and sensitive measure of cortical activity and, consequently, the potential to increase the signal-to-noise ratio by limiting inputs from other unrelated brain regions and allow for a deeper understanding of the mechanisms underlying the behavioral patterns (Lloyd-Fox et al., 2010). Previous fMRI and EEG source localization studies have linked asymmetry of the dorsolateral prefrontal cortex (dlPFC), a brain region associated with planning and cognitive flexibility, with behavioral motivation tendencies, and suggest that dlPFC asymmetry is a major contributor to the frontal alpha asymmetry signal (Davidson, 2004; Smith et al., 2018). Asymmetries in other brain regions of interest, such as the parietal cortex, often associated with sensory integration, or across the whole hemisphere, may also contribute to behavioral motivation and psychological functioning (Fox, 1991; Stewart, Towers, et al., 2011). Thus, examining brain asymmetries across multiple regions and their associations with behavioral outcomes may advance our understanding of the cognitive and neural mechanisms underlying psychological constructs. The high spatial resolution offered by fNIRS allows for such examination.
Overall, the current study had three major objectives: Aim 1: Examine whether FA responses in infancy assessed using fNIRS differ at the group level among happy, angry, and fearful faces. Aim 2: Test if individual differences in FA measured in response to emotional faces is associated with emotional and behavioral problems in later childhood. Aim 3: Explore if and how asymmetries in other (i.e., non-frontal) areas of the cortex, particularly brain areas involved in different aspects of social information processing, including the inferior frontal cortex, medial temporal cortex, superior temporal cortex, and parietal cortex, are associated with later emotional and behavioral problems. Hypothesis 1: Infants will show greater relative left FA (indexing greater approach orientation) to happy and angry compared with fearful faces (Stewart, Coan, et al., 2011). Hypothesis 2: Infants who demonstrate greater relative right FA (indexing withdrawal motivation) responses to happy, angry, and fearful faces will exhibit increased internalizing (depression, anxiety) and stress (difficulties concentrating, somatic symptoms, mood changes) problems at 5 years of age (Buss et al., 2003; Diaz & Bell, 2012; Feng et al., 2012; Peltola et al., 2014; Schmidt et al., 2010; Thibodeau et al., 2006). Given the lack of work examining the association between FA and externalizing (attention-deficit/hyperactivity problems [ADHD], oppositional defiant disorder [ODD]) problems, we did not have a specific directional hypothesis for this association (Ellis et al., 2017; Peltola et al., 2014; Smith & Bell, 2010). Aim 3 exploratory analysis: We hypothesized that FA localized to the dlPFC would be association with behavioral outcomes. Given that little work has systematically examined the associations of asymmetries in cortical regions other than frontal with behavioral outcomes, we did not have specific hypotheses regarding these brain–behavior associations.
The current study leverages fNIRS capabilities to add to the existing EEG FA literature by providing information about where the FA signal is localized and how the FA response differs across emotional facial expressions. Additionally, this study is one of the first to assess the relation between FA patterns during infancy and emotional and behavioral problems in early childhood.
METHOD
Participants
Participants were recruited at infancy from a database of families in Boston, Massachusetts and the surrounding area who had volunteered for research following their infants' birth and were invited to participate in a larger longitudinal study of the development of emotion processing (data collection period: 2012–2021). Ninety children (n = 48 male sex assigned at birth; n = 42 female sex assigned at birth) were included in the final analytic sample of the current study. Children participated in a laboratory session in infancy (Mage = 7.63 months; SD = 3.01; range = 4.0–12.0 months; see age distribution histogram in Supplemental Materials, Figure S1), during which fNIRS data were acquired. When the children were 5 years of age (Mage = 62.05 months; SD = 2.04; range = 60.0–70.0 months), families were invited to complete a study assessment that included maternal report regarding their child's mental health status (n = 88 questionnaire completion; n = 71 clinical interview completion). Power analysis in G*Power indicated that this sample size (N = 90) was sufficient to detect small-to-moderate linear effect sizes (f2 > .09) at standard α = .05 with .80 power. Parents gave informed consent for their child to participate in accordance with the Declaration of Helsinki, and all procedures were approved by the relevant institutional review board. Participants received monetary compensation for participation.
Exclusion criteria
Exclusion criteria for enrollment in the larger cohort included known prenatal or perinatal complications, developmental delay, uncorrected vision difficulties, and neurological disorder or trauma. After enrollment, families were no longer followed and their data were excluded from analyses if the child was diagnosed with an autism spectrum disorder or if a genetic or other condition known to influence neurodevelopment was diagnosed (e.g., hydrocephalus, absence of seizures, brain tumor, maternal use of anticonvulsants, antipsychotics, opioids in pregnancy). Seventy-three additional children participated in both the infant and 5-year testing sessions but were excluded from the present analyses because (a) they refused to wear the fNIRS cap (n = 18), (b) their cap deviated beyond 1.5 cm from the correct placement (n = 16), (c) equipment failed (n = 6), (d) they failed to reach our pre-determined looking criterion (n = 11), or (e) channels in areas of interest did not pass quality thresholds applied during pre-processing (n = 22). This rejection criterion and attrition rate are similar to those in other infant fNIRS studies (Baek et al., 2021; Bayet et al., 2021; Farris et al., 2022; Filippetti et al., 2014; Kelsey et al., 2019, 2021; Lloyd-Fox et al., 2015). As displayed in Table 1, the final analytic sample was predominately Non-Hispanic White, and parents were highly educated. In a series of chi-square and independent samples t-tests, no differences were found between included and excluded children on sociodemographic characteristics or outcome variables of interest (all p-values > .057). There was, however, a difference in temperament such that included infants (M = 3.14, SD = 0.78, variance = .61, range [1.40–5.55]) had lower ratings compared with excluded infants (M = 3.49, SD = 0.71, variance = .51, range [1.64–5.06]) on negative emotionality, t(161) = 3.00, p = .003, d = .472. There were no differences between groups on the temperament domains of orienting/regulation, t(161) = −1.74, p = .083, or surgency/extraversion, t(161) = 0.26, p = .793.
| Mean/n (SD/%) | |
|---|---|
| Sociodemographic characteristics | |
| Age in months at infant fNIRS testing session | 7.63 (3.01) |
| Age in months at follow-up psychological assessment | 62.05 (2.04) |
| Female sex assigned at birth | 42 (46.7%) |
| Race | |
| White | 75 (83.3%) |
| Black | 1 (1.1%) |
| Asian | 3 (3.3%) |
| Multiracial | 10 (11.1%) |
| Did not respond | 1 (1.1%) |
| Parental education | |
| High school or general education diploma | 7 (7.8%) |
| Associates degree | 1 (1.1%) |
| Bachelor's degree | 26 (28.9%) |
| Graduate school degree | 56 (62.2%) |
| Covariates | |
| Maternal depression symptom score | 4.86 (4.38) |
| Infant negative emotionality score | 3.14 (.78) |
| Outcome measures | |
| CBCL T-scores (N = 88) | |
| Internalizing | 45.42 (9.79) |
| Externalizing | 42.98 (10.38) |
| Stress | 52.63 (4.85) |
| Depression | 52.36 (3.85) |
| Anxiety | 52.56 (5.63) |
| ADHD | 51.64 (3.94) |
| ODD | 53.13 (5.76) |
| DIPA anxiety diagnoses (N = 71) | |
| Met criteria for at least one lifetime anxiety disorder | 13 (18.3%) |
| Separation anxiety disorder | 6 (8.5%) |
| Social anxiety disorder (social phobia) | 7 (9.9%) |
| Generalized anxiety disorder | 3 (4.2%) |
- Note: DIPA lifetime anxiety disorder categories are not mutually exclusive.
- Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CBCL, Child Behavioral Checklist; DIPA, Diagnostic Infant Preschool Assessment; fNIRS, functional near-infrared spectroscopy; ODD, oppositional defiant disorder.
Infant fNIRS testing session
fNIRS procedure
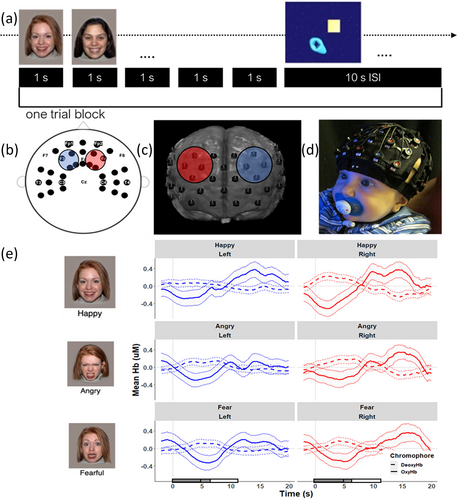
Infants sat on their caregiver's lap in a quiet, dimly lit room, approximately 60 cm from the screen (17-in. monitor). Caregivers wore visors to block their view of the presented stimuli to prevent inadvertent signaling or responding and were asked to refrain from talking during the experiment. The experimental paradigm was presented using E-Prime 2.0 (Psychological Software Products; Schneider et al., 2002). To ensure infants looked at the screen, each experimental trial was started manually by the experimenter when the infant was observed to be attending. The entire experimental session lasted approximately 15 min.
Stimuli
Color photographs of females expressing happiness, anger, and fear were chosen from a validated stimulus set (NimStim; Tottenham et al., 2009). Races of the models were matched to the mother's self-reported race. In each block, five faces of different women with the same facial expression (e.g., all angry) were presented for 1 s each with a break of 0.2–0.4 s between photographs (total trial length = 6–7 s). A 10-s non-social interstimulus interval video featuring colorful shapes moving across the screen was then shown. The visual angle of face stimuli was approximately 14.3° high by 12.2° wide. A total of 30 possible trials (10 per emotion: happy, angry, fearful) were presented, and trial order was counterbalanced across infants.
Data acquisition
Infants' fNIRS data were recorded using a Hitachi ETG-4000 continuous-wave system. The fNIRS method quantifies concentration changes of oxyHb and deoxyHb at a probing depth of approximately 1.5 cm below the cerebral cortex surface (for a review of this method see Lloyd-Fox et al., 2010). Data were recorded at a sampling rate of 10 Hz at 695 and 830 nm. The Hitachi ETG-4000 continuous-wave system used 14 source-detector pairs (46 channels) approximately 3 cm apart (Figure 1). At the beginning of the visit, the infant's head size was measured, and an appropriately sized cap was selected. Caps were placed with reference to anatomical landmarks, and photos were taken to document cap placement.
Excluding non-usable data
Cap placement photos were reviewed by trained research assistants. If the cap deviated more than 1.5 cm from the correct placement, the infant's data were excluded. In addition, infants' attention to the screen during the fNIRS testing session was coded offline from video recordings. Trials were only included if the infant looked to the screen for more than 60% of the full trial length. Reliability coding completed on 21 (23.3%) of the videos showed a high level of agreement between coders on the amount of time the infant looked at the screen (84.0% agreement) and on the decision to include/exclude trials (94.0% agreement). To be included in the current analyses, the infant had to contribute at least three trials with adequate attentiveness per emotion condition. On average, infants contributed data for a total of 20.79 trials, SD = 5.32 (Mhappy = 6.99, SD = 1.98; Mangry = 6.91, SD = 2.05; Mfear = 6.89, SD = 1.86). Thus, within this design, infants were exposed to twice as many negative emotions (anger and fear) as positive emotions (happy).
Average hemodynamic response calculation
Functional near-infrared spectroscopy data were preprocessed using Homer2 and custom MATLAB scripts (following guidelines put forth by Powell, 2020). First, raw intensity data were converted to optical density (OD) units (hmrIntensity2OD). Next, channels were pruned if they had (1) mean intensities outside the system recommended values (enPruneChannels; dRange = [1, 4], SNRthres = 0, SDrange = 0, 45), (2) high-frequency amplitude changes with over 90% of time points marked as motion (hmrMotionArtifactByChannel_indLamba; tMotion = 1.0, tMask = 1.0, Std Thresh = 100, Amp Thresh = 0.1), or (3) a large spike in the signal (hmrMotionArtifactByChannel; Amp Thresh = 2.0). Then, a flexible targeted Principal Component Analysis with up to three iterations (hmrMotionCorrectPCArecurse; tMotion = 1.0, tMask = 1.0, Std Thresh = 100, Amp Thresh = 0.1, nSV = 0.97) was used to correct for motion artifacts. Following motion correction, time points that remained contaminated by noise were excluded from the time courses (hmrMotionArtifactByChannel; tMotion = 1.0, tMask = 1.0, Std Thresh = 100, Amp Thresh = 0.1), and then a band-pass filter (third-order Butterworth) was applied using a 0.3 Hz low-pass filter and a high-pass filter of 0.02 Hz (for fNIRS studies with similar ages and filter parameters, see Kelsey et al., 2019; Krol et al., 2021). OD data were then converted to concentration changes using the modified Beer–Lambert law using a path length factor commonly used during infancy of 5 (Bayet et al., 2021; Cope & Delpy, 1988; Duncan et al., 1996; Pirazzoli et al., 2019). On average 9.76 channels (SD = 8.26) were rejected per child. Data were then visually inspected and rejected if artifacts remained in the data. OxyHb and deoxyHb concentration changes were calculated by averaging the hemodynamic response across a 5- to 11-s time window (see the Supplementary Results section for an analysis using a larger time window). The time window was chosen based on peak responding across all channels, and similar windows have been used in previously published manuscripts using these data (see Bayet et al., 2021; Porto et al., 2020; Ravicz et al., 2015). Note, prior work published using this dataset has focused on emotion discrimination and functional near-infrared spectroscopy data processing in the infancy period.
Anatomical localization
The left and right dlPFC regions were created by selecting 10–20 reference points (F1/F2 and F3/F4) and mapping channels onto infant brain space. To localize channels, age-appropriate and head circumference matched MRIs were chosen from a database (Richards et al., 2016; Sanchez et al., 2012). In line with previous work, cap configurations and cap placement photographs were used to approximate the source-detector locations on each individual MRI (Lloyd-Fox et al., 2014). Photon propagation modeling (Fang, 2010) estimated diffuse optical tomography sensitivity functions from each channel (Fu & Richards, 2020; Lloyd-Fox et al., 2014; Okamoto & Dan, 2005). Regions of interest were specified based on the averaged localization of each channel over the group of MRIs and labeled using the LONI atlas (Fillmore et al., 2015). Figure 1 displays the average location estimation of the region of interest (channels are indicated by circles) on an average MRI 7.5-month-old template (Richards et al., 2016).
Computing FA scores
Drawing from the extant EEG literature, hemispheric asymmetry scores (FA) were created by subtracting left dlPFC response from right dlPFC response (i.e., right–left). Thus, positive scores indicate greater relative right asymmetry (withdrawal motivation; likened to lower relative right alpha power using EEG), and negative scores indicate greater relative left asymmetry (approach motivation; likened to lower relative left alpha power using EEG).
Five-year follow-up assessment of child mental health
Child Behavior Checklist 1½–5
The Child Behavior Checklist 1½–5 (CBCL 1½–5) is a 99-item questionnaire with high validity and reliability used to assess emotional and behavioral problems in young children (Achenbach & Rescorla, 2000). Questionnaires were completed by the child's mother online using the REDCap survey platform (Harris et al., 2009, 2019) at home or in person during a laboratory visit. Mothers were asked to use a 3-point scale to rate if and how often particular behaviors occurred over the past 6 months (0 = not true, 1 = somewhat or sometimes true, 2 = very true or often true). T-scores were calculated for the two broad-band scales of Internalizing Problems and Externalizing Problems; for the Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented subscales of Depressive, Anxiety, ADHD, and ODD Problems; and for the Stress Problems subscale.
The Diagnostic Infant Preschool Assessment
Mothers were invited to complete the Diagnostic Infant Preschool Assessment (DIPA), a semi-structured clinical interview for caregivers of young children, which was administered to obtain ratings of lifetime and current psychiatric disorders (Scheeringa & Haslett, 2010). The DIPA has demonstrated reliability and validity in assessing clinical symptoms in research with very young children (Scheeringa & Haslett, 2010) and was developed such that it can be administered by trained research staff without specific clinical mental health experience or training. The DIPA was administered as an interview by trained research staff, supervised by a licensed clinical psychologist (author MBE) who met with the interviewers in biweekly group supervision meetings to discuss cases. The DIPA (version 7/12/14) assesses a variety of DSM-5 disorders and other problems, including anxiety disorders, mood disorders, and externalizing disorders (ADHD, ODD, conduct disorder), each in a self-contained module. These data were drawn from a larger longitudinal study aimed at identifying early risk markers for anxiety disorders, and anxiety disorders is the most well-represented type of disorder in the full sample. For the purposes of analysis, children were coded yes/no (1/0) as to whether they met criteria for separation anxiety disorder, social anxiety disorder/social phobia, and/or generalized anxiety disorder in their lifetime.
Data analysis
Covariates
To determine covariates that should be included in analyses, we tested for associations among the main study variables and age at infant fNIRS assessment, sex, infant temperament factors (negative emotionality, surgency, and orienting/regulation) assessed using the Infant Behavior Questionnaire Revised Short Form (Gartstein & Rothbart, 2003), and maternal depressive symptoms assessed using the Beck Depression Inventory-IA (Beck & Steer, 1987) at age 5 years. For further information on these measures, see Supplementary Methods. Any variable that was associated with the study outcome at p < .05 was included as a covariate in adjusted analyses.
Data analysis plan
In preliminary analyses, Spearman's rank correlations were conducted to test for potential covariates. To address Hypothesis 1, paired-samples t-tests were conducted to test for group-level differences between left versus right dlPFC responses for happy, angry, and fearful facial expressions and for differences in FA scores between happy, angry, and fearful facial expressions to test our hypothesis that infants would show greater relative left FA to happy and angry compared with fearful faces. To address Hypothesis 2, regression analyses were conducted to examine associations between infant FA and emotional and behavioral problems at age 5 years to test our hypothesis that greater relative right FA to happy, angry, and fearful faces would be associated with greater internalizing symptoms and FA would be associated with externalizing symptoms (non-directional hypothesis). Regression analyses were conducted because the outcome variables (Internalizing and Externalizing T-scores) were normally distributed. The regression model fit (for the unadjusted model) and parameters (for unadjusted and adjusted models) are reported. Hurdle models were used to assess the relation between FA patterns and scores on the CBCL problem subscales. Hurdle models were conducted for the CBCL subscales to account for the zero-inflated distribution of the subscale data; raw subscale scores were used due to the distribution assumptions of the hurdle model (i.e., the scale must start at 0). Moreover, since t-scores but not raw scores take into account the child's age and gender, both age and gender were added as covariates in analyses using the raw CBCL scores. Binary logistic analyses were conducted to examine the association between infant FA and lifetime anxiety diagnoses made using the DIPA. To address Exploratory Aim 3, a series of Spearman's correlation analyses were conducted to test for associations between asymmetries in non-frontal cortical areas and emotional and behavioral problem scores at 5 years to explore whether any asymmetry findings were restricted to the frontal region or found in other brain regions.
Multiple comparisons corrections were applied to all analyses using false discovery rate (q-value < .05 is considered significant). The main analyses presented focus on oxyHb responses; additional bivariate correlation analyses assessing deoxyHb responses are included in Figure S4.
RESULTS
Descriptive statistics
An overview of the sample sociodemographic characteristics and descriptive statistics for the covariate and outcome variables are presented in Table 1.
Preliminary analyses to determine covariates for inclusion in adjusted models
A series of Spearman's rank correlations were conducted between age during the infant testing session and study variables. There were no significant associations found between infant age or infant sex and happy FA, angry FA, or fear FA (see Figure 2 for more information). Therefore, infant age and sex were not included as covariates in adjusted analyses (see Supplementary Results for more information).
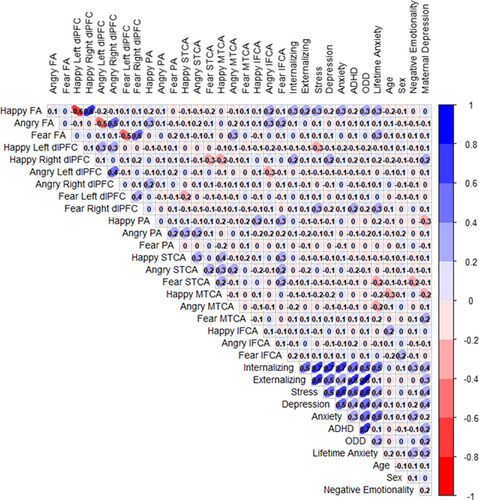
Spearman's rank correlation analyses revealed associations between infant negative emotionality, maternal depressive symptoms at 5 years, and the main study variables (see Figure 2 for details). Therefore, we included these covariates in all adjusted analyses. There was also an association between infant orienting/regulation and ADHD (rs = .22, p = .041); therefore, orienting/regulation was included as a covariate in all analyses with ADHD as the outcome.
Hypothesis 1a: Assessing group-level FA lateralization patterns
Paired samples t-tests did not reveal differences between left versus right dlPFC responses to happy (t(89) = 0.035, p = .972), angry (t(89) = 0.01, p = .989), or fearful (t(89) = −0.59, p = .555) faces.
Hypothesis 1b: Assessing condition effects on FA in infancy
Paired samples t-tests did not reveal differences in infant FA scores to happy versus angry faces (t(89) = 0.021, p = .983), happy versus fearful faces (t(89) = 0.40, p = .688), or angry versus fearful faces (t(89) = −0.44, p = .663).
Hypothesis 2a: FA in infancy and the development of emotional and behavioral problems at age 5 years
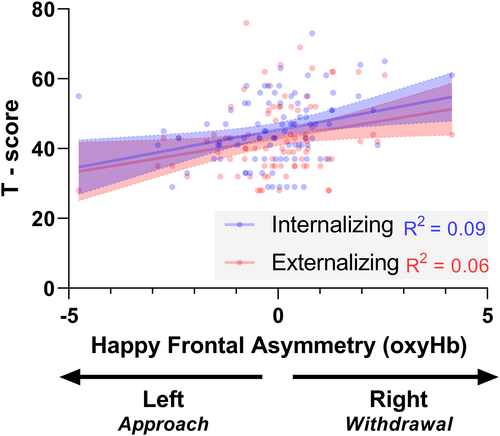
In a multivariate regression analysis with happy FA as the independent variable and both externalizing and internalizing problems T-scores as the outcome variables, happy FA was associated with multivariate outcomes, F(2, 85) = 4.46, p = .014, η2 = .095. Specifically, greater happy FA (i.e., a more right lateralized response) was positively associated with both internalizing problems (unadjusted B = 2.26, SE = .80, p = .006, q-value = .012, η2 = .085; adjusted B = 1.99, SE = .74, p = .009, q-value = .018, η2 = .078) and Externalizing Problems (unadjusted B = 2.01, SE = .86, p = .022, q-value = .022, η2 = .059; adjusted B = 1.80, SE = .85, p = .038, q-value = .038, η2 = .050; see Figure 3; Table S1 for full regression models). In separate multivariate regression analyses with angry FA and fear FA independent variables and both externalizing and internalizing problems T-scores as the dependent variables, no associations were found (adjusted and unadjusted p-values > .26; see Table S2 and S3 for full-regression models).
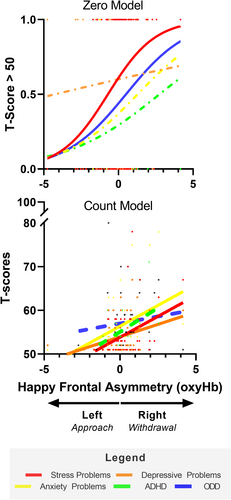
To further delineate the association between happy FA in infancy and emotional and behavioral problems at age 5 years, a series of hurdle models with happy FA scores as the independent variables and Stress Problems and the DSM-oriented subscales as the dependent variables were conducted. As shown in Table 2 and Figure 4, greater happy FA (i.e., a more right lateralized response) was associated with increased levels of stress problems, depressive symptoms, anxiety, and ODD problems. All associations between angry FA and fear FA in infancy and behavioral scores at age 5 did not reach significance (all p-values > .065).
| Hurdle model | Stress problems | Depressive problems | Anxiety problems | ADHD | ODD |
|---|---|---|---|---|---|
| Unadjusted model fit (AIC) | 265.84 | 281.93 | 363.47 | 386.60 | 383.01 |
| Unadjusted log-likelihood (df = 9) | −123.9 | −132.0 | −172.7 | −184.3 | −182.5 |
| Unadjusted count model parameters |
B = .35 SE = .15 p = .022 |
B = .25 SE = .10 p = .015 |
B = .27 SE = .10 p = .005 |
ns | ns |
| Unadjusted zero hurdle model parameters |
B = .64, SE = .23, p = .005 |
ns | ns | ns |
B = .54, SE = .22, p = .013 |
| Adjusted model fit (AIC) | 257.05 | 276.42 | 360.98 | 389.78 | 381.59 |
| Adjusted log-likelihood (df = 13) | −115.5 | −125.2 | −167.5 | −179.9 | −177.8 |
| Adjusted count model parameters |
B = .38 SE = .16 p = .017 |
ns |
B = .23 SE = .09 p = .008 |
ns | ns |
| Adjusted zero hurdle model parameters |
B = .78 SE = .26 p = .003 |
ns | ns | ns |
B = .58 SE = .24 p = .016 |
- Note: All models included the covariates of age and sex since the analyses were conducted on the raw scores (as opposed to the t-scores). The adjusted parameters and adjusted odds ratio scores are for the models that include covariates (infant negative emotionality and maternal depressive symptoms in children aged 5 years). The adjusted model with ADHD symptoms as the outcome variable includes infant orienting/regulation temperament scores as an additional covariate. Values in bold survived FDR multiple comparisons correction (q-value < .05).
- Abbreviations: ADHD, attention deficit/hyperactivity disorder problems; AIC, Akaike information criterion; CBCL, Child Behavior Checklist; DSM, Diagnostic and Statistical Manual of Mental Disorders; ODD, oppositional defiant disorder problems.
Hypothesis 2b: Associations between FA and diagnoses made using the DIPA
Consistent with the findings using the CBCL, happy FA was positively associated with the likelihood of having a lifetime anxiety diagnosis per the DIPA, χ2(1, N = 71) = 7.12, p = .008, R2 = .16 (unadjusted B = .80, SE = .34, p = .018, odds ratio 95% CI [1.15–4.29]; adjusted B = .83, SE = .37, p = .024, odds ratio 95% CI [1.11–4.72]). In contrast to the findings using the CBCL, fear FA was positively associated with the likelihood of having a lifetime anxiety diagnosis per the DIPA, χ2(1, N = 71) = 6.09, p = .014, R2 = .13 (unadjusted B = .92, SE = .41, p = .023, odds ratio 95% CI [1.14–5.58]; adjusted B = .91, SE = .44, p = .039, odds ratio 95% CI [1.05–5.90]; see Table S4 for full regression models). However, angry FA was not significantly associated with the likelihood of having a lifetime anxiety disorder (adjusted and unadjusted p-values > .159).
Exploratory Aim 3: Localizing the association between infant FA and childhood emotional and behavioral problems
In brief, no associations were found between asymmetry scores for any other brain regions (i.e., superior temporal cortex, medial temporal cortex, parietal cortex, inferior frontal cortex) and child CBCL scores (all p-values > .05), providing support for a localized effect in the dlPFC (see Figure 2 for correlation results and Figure S3 for waveforms).
DISCUSSION
This study is one of the first to use fNIRS to examine whether patterns of FA, that is, the difference in brain activity between left versus right frontal areas, assessed in response to viewing emotional facial expressions during infancy, are associated with emotional and behavioral problems at 5 years of age. Our results showed that greater relative right FA to happy, but not to negative (fear, anger), emotion faces in infancy was associated with increased levels of internalizing and externalizing behavior problems in later childhood. Moreover, greater relative right FA to happy and fearful faces was associated with an increased likelihood of having a lifetime anxiety diagnosis by age 5 years. Furthermore, we assessed asymmetry across five cortical regions and found only asymmetry patterns localized in the dlPFC region were associated with later problem behaviors. These findings suggest that the assessment of FA in response to emotional stimuli may aid in the early detection of psychopathology.
Contrary to our hypothesis (Hypothesis 1) and prior adult work using EEG that has shown FA condition differences to emotional stimuli (Stewart, Coan, et al., 2011), we did not find differences at the group level for FA in response to happy, angry, and fearful emotional faces. However, at the individual level, FA scores to happy were more consistently associated with later outcomes compared with FA scores to negative emotions. One possible explanation for the lack of condition effects at the group level is the difference in neural responding indexed by EEG (i.e., neuronal activity) versus fNIRS (i.e., metabolic activity indexed by blood flow). In addition, it is possible that the lack of group differences is due to the region of interest selected for the present study (i.e., the localized [dlPFC] FA response when assessed using fNIRS vs. widespread brain contributions to FA when assessed using EEG). In addition, group differences may have been obscured by individual differences within the sample. For example, two recent studies (one using EEG and one using fNIRS) failed to find FA differences across conditions for the full sample but found group-by-condition effects (e.g., significant differences in FA across conditions for one group [autism spectrum disorder vs. neurotypical; risk allele vs. no risk allele for oxytocin receptor gene] but not the other; Krol et al., 2021; Lauttia et al., 2019). Moreover, a similar pattern was reported in a sample of 5-year-old children, where there were no differences in FA assessed using EEG at the group level between happy and sad films (but both were associated with increased activity [decreased power] compared with a non-social task period); yet, there were differences at the individual level, where only FA response to a happy (but not sad) film was associated with depressive symptoms (Feng et al., 2012). Alternatively, the lack of group-level differences between positive and negative stimuli may be a result of developmental mechanisms involved in the growing experience and understanding of emotion categories. Infants typically have less day-to-day exposure to negative emotional faces than happy faces; thus, fear and angry faces tend to be less familiar (Malatesta & Haviland, 1982). However, by the end of the first months of life, infants have had significant exposure to faces and show differential attentional and behavioral responding to emotional expressions, they may not fully understand these emotion categories until early childhood with the onset of language and use of emotion labels (Ruba & Repacholi, 2020). Therefore, group-level differences in FA to different emotion categories may not emerge until later in childhood. The development of emotion expression recognition in early life is important because appropriately recognizing and responding to emotional stimuli contribute to later psychological well-being (Grinspan et al., 2003; Izard, 2002; Izard et al., 2001; Malatesta & Haviland, 1982; Ruba & Repacholi, 2020). Overall, the current findings highlight the importance of assessing FA under specific contexts (similar to suggestions put forward by the “capability model,” whereby FA may represent the ability to regulate and respond to emotional cues (Coan et al., 2006)).
Our finding that increased relative right FA to happy faces was associated with increased internalizing problems is in line with our hypothesis (Hypothesis 2) and consistent with meta-analytic work with children and adults that have found moderate associations between relative right frontal alpha asymmetry and internalizing symptoms (Peltola et al., 2014; Thibodeau et al., 2006). In addition, prior correlational work in humans (Thibodeau et al., 2006) and experimental work with animals (Lyons et al., 2002) has shown that early adversity, a risk factor for internalizing symptoms, is associated with increased relative right FA. Notably, the association between happy FA and psychological outcomes was held even after controlling for infant temperament. One possible interpretation of this finding is that FA may be an independent predictor rather than an approximation for other co-occurring behaviors. We were also able to provide additional measurement and clinical validity by including a semi-structured clinical assessment, the DIPA. Here, we found that greater right FA to happy faces was associated with an increased likelihood of having a lifetime anxiety diagnosis by age 5 years. These results suggest that FA to emotional stimuli may be of clinical relevance, and more work should be done to test this association with larger samples and other clinical diagnoses.
We found little evidence that infant FA responses to faces displaying negative emotion (fear, anger) were associated with childhood behavioral problems. Specifically, neither infant FA in response to angry or fearful faces was associated with internalizing or externalizing symptoms on the CBCL. These findings run contrary to prior fNIRS work that has shown neural responses to negative emotions to be especially relevant for behavioral outcomes (Grossmann et al., 2018) and to FA emotion challenge work using EEG with adults that have shown that increased relative right FA in both positive and negative emotion contexts is associated with depression (Stewart, Coan, et al., 2011). However, in line with previous findings, we found that greater right FA in response to fear was associated with increased likelihood of being diagnosed with a lifetime anxiety disorder by age 5 years. Given that both happy and fear FA were associated with increased risk for a lifetime anxiety disorder diagnosis, it may be that greater right FA (a withdrawal response) to social stimuli more generally, and not specific affective contexts, confers risk. More work is needed that includes specific controls (e.g., a neutral face) to elucidate the specific contexts in which infant FA is associated with later child emotional and behavioral problems and whether predictors of clinical levels of psychological problems differ from those of subclinical levels.
We further found that increased relative right FA in response to happy faces was associated with greater externalizing behaviors (Hypothesis 2). This finding may lend support to the interpretation that FA is a shared neural underpinning of both internalizing and externalizing psychopathology. However, the findings to date between FA and externalizing symptoms have been mixed, with some showing no association and others showing increased relative left FA, thought to reflect anger and approach behaviors, is correlated with greater externalizing behaviors (Ellis et al., 2017; Peltola et al., 2014; Smith & Bell, 2010). One potential reason why our study may differ from previous studies is the use of emotional stimuli. FA has mostly been examined under resting-state conditions. Therefore, more work is needed examining associations between FA assessed in an emotional context and externalizing disorders. In addition, more longitudinal work with multiple assessments of FA and outcome behaviors across childhood is needed to understand the stability of early FA responses and how FA responses to emotional stimuli across infancy and early childhood may contribute to both internalizing and externalizing disorders.
Another reason why the present findings may differ from previous findings is that the nature of the association between FA and psychopathology may change with age. Compared with similar prior work, the infants in the present study were younger during the FA assessment (4–12 months compared with 10 and 24 months) and older during the psychological outcomes assessment (5 years compared with 30 months; Smith & Bell, 2010). The latter distinction is particularly important given that 5 years of age is a risk period for the onset of some anxiety and other behavioral problems, as this is the time when children typically begin formal schooling and certain problem behaviors are first identified in the face of new environments and stressors (Cartwright-Hatton et al., 2006; Headley & Campbell, 2011; Liu, 2004). Of note, we did not find associations between age at the infancy assessment and FA scores for either the happy or negative emotion faces. This appears to be in line with prior work showing that FA is relatively stable across development (Brooker et al., 2017). However, this relative stability is surprising when considering the affective context of the stimuli and a large number of growth infants have in terms of emotion perception (Ruba & Repacholi, 2020). Overall, more longitudinal work assessing the stability and change of these brain-behavior patterns over time and across contexts is needed. In addition, future work should test the stability across a larger time frame (e.g., when children begin to understand and use emotion labels).
To further understand the specificity between FA and psychological outcomes, we tested whether FA was associated with heightened levels of symptomology for specific symptom subscales. Here, the largest effect size was found for the relation between FA and stress problems (e.g., difficulty concentrating, somatic symptoms, emotional lability), and the direction of effects was in line with our hypothesis and prior work. For example, in infants, greater relative right FA has been associated with heightened cortisol levels, a putative marker of stress (Buss et al., 2003). Therefore, the present findings complement prior biological work in showing associations between increased right-lateralized frontal brain activity and later maladaptive stress responding. Furthermore, results from our supplementary analyses suggest that the association between FA and clinical anxiety may be particularly relevant for social anxiety disorder, a finding which should be pursued in future research with larger clinical samples.
We were also interested in testing if asymmetries in non-frontal areas of the brain were associated with behavior problems (Exploratory Aim 3). In line with previous fMRI and source localization EEG studies, only asymmetry patterns localized to the dlPFC, an area often associated with cognitive flexibility and planning, and not in superior temporal cortex, medial temporal cortex, inferior frontal cortex, or parietal cortex, were related to later emotional and behavioral problems (Davidson, 2004; Smith et al., 2018; Spielberg et al., 2011). Thus, our findings suggest a role of the dlPFC beginning in early life in child's psychological health. Further research, particularly in early development, should confirm this localized specificity and use complementary methods, such as concurrent EEG and fNIRS recordings, to better understand the relations between the different modalities.
Finally, in supplemental analyses, we did not detect differences in FA patterns for deoxyHb. This lack of findings for the deoxyHb chromophore is not surprising given that absence of deoxyHb effects is common in infant fNIRS studies (Cristia et al., 2013) and that the oxyHb response has greater reliability and variability compared with the deoxyHb response (Dravida et al., 2018).
Limitations of this study should be acknowledged. The present study did not have control conditions, such as a neutral face or nonsocial condition. Therefore, we were unable to compare FA in response to emotional faces versus neutral faces, which would have helped elucidate the role of FA to specific emotional contexts versus social stimuli more generally. Similarly, this study was unable to disentangle the contributions of FA to faces/social stimuli from non-social stimuli or resting condition, the latter of which has formed the basis of the majority of work in this area to date. Therefore, future work should include both nonsocial and neutral face conditions. Participants were predominately White and most parents were highly educated, which may be, in part, a consequence of using a volunteer database as the primary recruitment method. Thus, the generalizability of the findings may be limited and should be replicated in more diverse samples. Additionally, this was a relatively low-risk community sample, with most children exhibiting symptoms below clinical thresholds at age 5. Future work in clinically high-risk samples should be considered to determine the validity of this method for predicting clinically significant outcomes. However, even in this low-risk sample, FA in infancy was able to provide some level of association for mental health status 4 years later. From a prevention standpoint, developing very early biomarker assessments capable of identifying young children at risk for developing psychological disorders prior to the emergence of symptoms is critical. This study also had several strengths, including its novel use of fNIRS, large sample size for a study of its kind, and longitudinal design.
Humans must continuously manage responses to positive, negative, and ambiguous contexts, with one's response tendencies potentially having long-term consequences for mental health. The current study suggests that having greater relative right FA (reflective of a withdrawal response) to positive stimuli, particularly localized to the dlPFC, may serve as a generalized marker for elevated risk for later psychopathology. Thus, FA responses observed in infancy may be informative for the early detection and prevention of later psychopathologies.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Mental Health (R01 MH078829; to CAN and MBE) and a Tommy Fuss Center Innovation Award from the Tommy Fuss Center for Neuropsychiatric Disease Research at Boston Children's Hospital (to MBE). This work was also supported by the National Institute of Child Health and Human Development (F32 HD105312-01A1; to CMK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health of the National Institutes of Health. Study data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at Boston Children's Hospital (Harris et al., 2009). REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources. We acknowledge Lindsey Powell and Hilary Richardson for developing the Matlab scripts used to process the fNIRS data. In addition, we would like to thank the emotion project team, including Katherine Vincent, Rachel Kwon, Kelsey Quigley, and Jebediah Taylor, for their contributions to this project. We thank all of the study families for their participation.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
The data, analytic code, and materials necessary to reproduce the analyses presented here are not publicly accessible. The analyses presented here were not preregistered. The data that support the findings of this study are available from the corresponding author upon reasonable request.