Rapid Icm/Dot T4SS Inactivation Prevents Resuscitation of Heat-Induced VBNC Legionella pneumophila by Amoebae
Funding: This work was supported by Geberit International AG.
ABSTRACT
Legionella pneumophila, the causative agent of Legionnaires' disease, employs the Icm/Dot Type IV secretion system (T4SS) to replicate in amoebae and macrophages. The opportunistic pathogen responds to stress by forming ‘viable but non-culturable’ (VBNC) cells, which cannot be detected by standard cultivation-based techniques. In this study, we document that L. pneumophila enters the VBNC state after exposure to heat stress at 50°C for 30 h, at 55°C for 5 h or at 60°C for 30 min, while still retaining metabolic activity and intact cell membranes. Resuscitation of heat-induced VBNC L. pneumophila neither occurred in amoebae nor in macrophages. VBNC L. pneumophila showed impaired uptake by phagocytes, formation of Legionella-containing vacuoles (LCVs), and Icm/Dot-dependent secretion of effector proteins. The T4SS was rapidly inactivated already upon exposure to 50°C for 3–5 h, while the bacteria were still culturable. The Legionella quorum sensing (Lqs)-LvbR network is implicated in VBNC induction, since the ∆lvbR and ∆lqsR mutant strains showed a more pronounced heat sensitivity than the parental strain, and the ∆lqsA mutant was less heat sensitive. Taken together, our results reveal that heat exposure of L. pneumophila rapidly inactivates the Icm/Dot T4SS before the VBNC state is induced, thus impairing resuscitation by amoebae.
Graphical Abstract
The environmental bacterium Legionella pneumophila employs a Type IV secretion system (T4SS) to replicate in amoebae and macrophages in a unique ‘Legionella-containing vacuole’ (LCV), thereby causing Legionnaires' disease. The opportunistic pathogen responds to stress by (reversibly) forming ‘viable but non-culturable’ (VBNC) cells, which are metabolically active but non-culturable under standard conditions. In this study, we reveal that heat-induced VBNC L. pneumophila lack a functional T4SS, do not form LCVs, and cannot be resuscitated by phagocytes.
Abbreviations
-
- CCCP
-
- carbonyl cyanide m-chlorophenylhydrazone
-
- c-di-GMP
-
- cyclic-di-guanylate mono phosphate
-
- DAPI
-
- 4′,6-diamidin-2-phenylindol
-
- DiBAC
-
- Bis-(1,3-dibutylbarbituric acid)pentamethine oxonol
-
- FDA
-
- fluorescein diacetate
-
- Icm/Dot
-
- intracellular multiplication/defective organelle trafficking
-
- IFC
-
- imaging flow cytometry
-
- LAI-1
-
- Legionella autoinducer-1
-
- LCV
-
- Legionella-containing vacuole
-
- LD
-
- Live-or-Dye
-
- Lqs
-
- Legionella quorum sensing
-
- LvbR
-
- Legionella virulence and biofilm regulator
-
- MOI
-
- multiplicity of infection
-
- NO
-
- nitric oxide
-
- PI
-
- propidium iodide
-
- PMF
-
- proton motive force
-
- T4SS
-
- type IV secretion system
-
- TO
-
- thiazole orange
-
- VBNC
-
- viable but non-culturable
1 Introduction
Legionella pneumophila, the causative agent of Legionnaires' disease, is a Gram negative, obligatory aerobic bacterium that inhabits environmental freshwater as well as artificial water systems (Newton et al. 2010; Hilbi and Buchrieser 2022). The facultative intracellular bacterium infects human alveolar macrophages after the inhalation of contaminated aerosols, which can lead to a severe and potentially fatal pneumonia, especially in elderly and/or immuno-compromised individuals (Fields, Benson, and Besser 2002; Mondino et al. 2020). The virulence of the opportunistic pathogen L. pneumophila crucially depends on the Icm/Dot (intracellular multiplication/defective organelle trafficking) Type IV secretion system (T4SS), which translocates over 300 ‘effector proteins’ into the host cell to manipulate various host processes and establish a unique replication-permissive compartment, called the Legionella-containing vacuole (LCV) (Asrat et al. 2014; Finsel and Hilbi 2015; Personnic et al. 2016; Qiu and Luo 2017; Steiner, Weber, and Hilbi 2018; Lockwood et al. 2022).
The genetically tractable social soil amoeba Dictyostelium discoideum has been instrumental in elucidating the molecular mechanism of LCV formation (Solomon and Isberg 2000; Steinert and Heuner 2005; Weber and Hilbi 2014; Swart et al. 2018), in particular by using dually fluorescence-labelled amoebae (Hüsler et al. 2023; Vormittag, Hüsler, et al. 2023). LCV formation is a complex process that includes the phosphoinositide lipid conversion from phosphatidylinositol 3-phosphate (PdtIns(3)P) to PdtIns(4)P (Weber et al. 2006, 2018; Weber, Wagner, and Hilbi 2014; Swart and Hilbi 2020), interaction with the endoplasmic reticulum (ER) through membrane contact sites (MCS) (Vormittag, Ende, et al. 2023; Vormittag, Hüsler, et al. 2023) and recruitment of lipid droplets (Hüsler et al. 2021, 2023; Hüsler, Stauffer, and Hilbi 2023).
L. pneumophila determines interactions with host cells not only through the Icm/Dot T4SS but also by small molecule signalling through nitric oxide (NO) (Michaelis, Chen, et al. 2024) and the α-hydroxyketone compound LAI-1 (Legionella autoinducer-1) (Spirig et al. 2008). LAI-1 is produced and detected by the Lqs (Legionella quorum sensing) system (Tiaden et al. 2007; Tiaden, Spirig, and Hilbi 2010; Hochstrasser and Hilbi 2017), which among many other processes also regulates the emergence of antibiotic-tolerant, non-growing L. pneumophila ‘persisters’ in macrophages and amoebae (Personnic et al. 2019; Striednig et al. 2021). The Lqs system and in particular the autoinducer synthase LqsA determine the portion of non-growing versus growing bacteria in amoebae, and therefore, quorum sensing regulates L. pneumophila persistence (Personnic et al. 2019; Striednig and Hilbi 2022; Michaelis, Gomez-Valero, et al. 2024). The Lqs system and the transcription factor LvbR (Legionella virulence and biofilm regulator) also regulate the emergence of L. pneumophila persisters in sessile microcolonies and biofilms (Personnic, Striednig, and Hilbi 2021).
Many bacteria including L. pneumophila respond to a variety of stresses by entering a dormant ‘viable but non-culturable’ (VBNC) state (Oliver 2010; Liu et al. 2023). Unlike dead cells, bacteria in the VBNC state are still alive (but might be damaged), have an intact cell membrane, maintain metabolic activity, and remain transcriptionally and translationally active. However, they can no longer be cultivated on ‘standard’ laboratory media, which—along with their capacity to ‘resuscitate’ to a culturable state under suitable conditions—complicates their detection and risk assessment (Kirschner 2016; Liu et al. 2023). Viability dyes, often based on propidium iodide (PI) staining, are commonly used to differentiate between live, dead and VBNC bacteria. However, these dyes do not fully account for the physiological state of the bacteria and do not account for the bacteria's potential to resuscitate and possibly cause disease (Liu et al. 2023). VBNC bacteria are likely physiologically distinct from antibiotics-induced persister cells, representing a subpopulation of non- or slow-growing bacteria, which can be cultivated (Lewis 2007; Brauner et al. 2016).
The existence of VBNC L. pneumophila was originally described more than 30 years ago (Paszko-Kolva et al. 1991; Paszko-Kolva, Shahamat, and Colwell 1993). VBNC L. pneumophila can be induced through several stressors, such as starvation (Steinert et al. 1997; Al-Bana, Haddad, and Garduno 2014; Dietersdorfer et al. 2018), exposure to disinfection agents (Garcia et al. 2007; Alleron et al. 2008; Ducret, Chabalier, and Dukan 2014) or heat treatment (Epalle et al. 2015; Cervero-Arago et al. 2019). For these conditions, the resuscitation of VBNC L. pneumophila has been reported upon exposure to Acanthamoeba castellanii (Steinert et al. 1997; Alleron et al. 2008; Al-Bana, Haddad, and Garduno 2014; Ducret, Chabalier, and Dukan 2014; Dietersdorfer et al. 2018; Cervero-Arago et al. 2019) or Acanthamoeba polyphaga (Garcia et al. 2007; Epalle et al. 2015; Nisar et al. 2023). The VBNC state might contribute to the epidemiological discrepancy between the inefficient culture-based identification of L. pneumophila in environmental samples and the increasing number of clinical cases (van Heijnsbergen et al. 2015; Kirschner 2016).
Although the VBNC form of L. pneumophila can be induced through several environmental stressors, heat-induced VBNC L. pneumophila are of particular interest, since temperature upregulation, also referred to as thermal disinfection, is a key strategy employed for controlling Legionella in artificial water systems (Kim et al. 2002; Cervero-Arago et al. 2015, 2019; Epalle et al. 2015). The VBNC state is induced in L. pneumophila in the temperature range between 50°C and 70°C, questioning the efficacy of common thermal disinfection strategies (Allegra et al. 2008; Cazals et al. 2022; Nisar et al. 2023).
The temperature influences the survival, growth and behaviour of Legionella species. L. pneumophila grows within the temperature range of 18°C to ca. 45°C, with linearly increasing growth rates from 18°C to the optimum at ca. 37°C, followed by a sharp drop to zero between 40°C and 50°C (Hochstrasser and Hilbi 2022). Components of the Lqs-LvbR network regulate growth onset and culture density at different temperatures and in different media (Hochstrasser and Hilbi 2022). In the current study, we sought to assess the role of the Icm/Dot T4SS and the Lqs-LvbR regulatory network in heat-triggered VBNC induction and/or resuscitation.
2 Results
2.1 Induction of VBNC L. pneumophila by Heat Treatment
While previous studies have shown that heat-treatment induces the VBNC state in L. pneumophila, the mechanisms of VBNC induction and resuscitation are poorly understood. To address these questions, we assessed the loss of culturability of L. pneumophila exposed to temperatures ranging from 50°C to 60°C, tested the metabolic activity as well as the membrane integrity of the non-culturable cells, and attempted to resuscitate the VBNC bacteria.
The L. pneumophila wild-type strain JR32 and the avirulent, Icm/Dot-deficient ∆icmT mutant were exposed to elevated temperatures in water baths constantly kept at the temperatures indicated (Figure 1A). Following this treatment, the bacteria consistently became non-culturable after exposure to 50°C for 30 h, 55°C for 5 h or 60°C for 30 min. As expected, the Icm/Dot T4SS did not play a role in the process. Next, we assessed the viability of VBNC L. pneumophila by flow cytometry. To this end, fluorescein diacetate (FDA), an esterase-reactive dye, was used to assess metabolic activity, while Live-or-Dye (LD), an amine-reactive dye, was used to assess the membrane integrity. Through quadrant gating, viable bacteria, that is cells with metabolic activity (FDA-positive cells) and intact membranes (LD-negative cells), were distinguished and quantified (Figure S1A). Compared to untreated L. pneumophila, heat-treatment reduced the number of metabolically active, membrane-intact bacteria by 30%–50%. In contrast, heat inactivation by an exposure to 95°C for 10 min led to a complete loss of metabolic activity and membrane integrity (Figure 1B). Under any of the conditions at 50°C–60°C, L. pneumophila JR32 was no longer culturable, and therefore, the bacteria are VBNC cells by definition (Figure 1C).
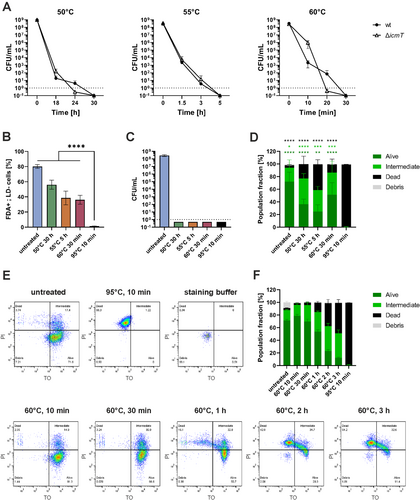
To further validate the data obtained with the two separate dyes, FDA and LD, the BD Cell Viability kit was used to assess bacterial viability. The commonly applied kit is based on staining with thiazole orange (TO) and PI, membrane-permeable or -impermeable dyes, respectively. Through flow cytometry and quadrant gating, ‘alive’ (TO-positive, PI-negative), ‘intermediate’ (TO-positive, PI-positive) and ‘dead’ (TO-negative, PI-positive) bacteria were distinguished and quantified (Figure S1B). Compared to the heat-inactivated control (100% dead), VBNC L. pneumophila were more similar to untreated bacteria, with over 60%–80% of the cells gating to the alive or intermediate quadrants (Figure 1D). The loss of culturability of the heat-treated bacteria was assessed via appropriate dilution plating (Figure S2A). To kinetically study the effects of heat treatment on L. pneumophila viability, the bacteria were exposed to 60°C and analysed by TO/PI membrane integrity staining at different time points within 3 h (Figure 1E). After 30 min at 60°C, the L. pneumophila population displayed the most pronounced VBNC characteristics (> 85% of bacteria gating to the alive or intermediate quadrants and complete loss of culturability) (Figure 1F, S2B). Upon prolongation of the exposure to 60°C, the number of PI-positive dead cells increased proportionally with the duration of the heat treatment. In summary, heat treatment of L. pneumophila (50°C, 30 h; 55°C, 5 h; or 60°C, 30 min) leads to the VBNC state as judged by the assessment of metabolic activity and membrane integrity based on fluorescent dyes.
2.2 The Lqs-LvbR System Regulates the Formation of VBNC L. pneumophila
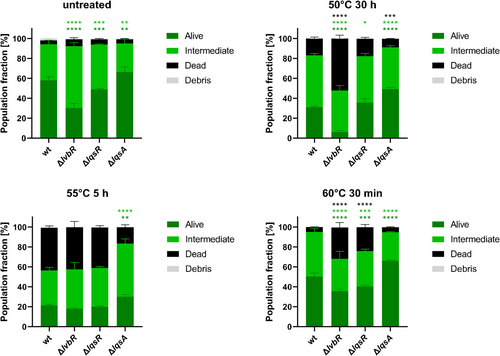
The Lqs-LvbR network controls temperature-dependent growth onset and cell density of L. pneumophila JR32 (Hochstrasser and Hilbi 2022). Compared to wild-type JR32, a strain lacking the response regulator gene lqsR (∆lqsR) shows a shorter lag phase at 30°C and reaches a higher cell density at 45°C, while the ∆lqsA mutant lacking the autoinducer synthase gene shows a longer lag phase but reaches a lower cell density. A ∆lvbR mutant strain deficient for the transcription factor LvbR resumes growth like the parental strain at 30°C but exhibits a significantly reduced cell density at 45°C (Hochstrasser and Hilbi 2022). Given these physiological phenotypes, we sought to assess the ∆lvbR, ∆lqsR and ∆lqsA mutant strains for their behaviour in heat-induced VBNC formation.
To quantify the viability of the ∆lvbR, ∆lqsR and ∆lqsA mutants, the membrane integrity was assessed by TO/PI staining and flow cytometry, distinguishing ‘alive’ (TO-positive, PI-negative), ‘intermediate’ (TO-positive, PI-positive) and ‘dead’ (TO-negative, PI-positive) bacteria through quadrant gating (Figure S1B). The staining profiles revealed that compared to the wild-type strain, the ∆lvbR and ∆lqsR mutants were more temperature sensitive at 60°C and in the case of ∆lvbR in particular also at 50°C, while the ∆lqsA mutant was more temperature resistant across the three temperatures tested (50°C, 55°C and 60°C) (Figure 2). Under the conditions used, the ∆lvbR, ∆lqsR and ∆lqsA mutant strains lost culturability (Figure S2C). Taken together, these results indicate that the Lqs-LvbR regulatory network is involved in the regulation of heat-induced VBNC formation of L. pneumophila.
2.3 Exposure to Amoebae Does Not Resuscitate Heat-Induced VBNC L. pneumophila
To assess the resuscitation of heat-induced VBNC L. pneumophila by amoebae, we quantified the intracellular replication of L. pneumophila producing fluorescent proteins. The fluorescence of L. pneumophila producing GFP, mCherry or mPlum was not affected by the conditions used to generate heat-induced VBNC bacteria (60°C, 30 min), indicating that the treatment did not compromise the stability of the fluorescent proteins (Figure S3A,B), while the bacteria were indeed in a VBNC state (Figure S3C).
GFP-producing VBNC L. pneumophila JR32 and—as negative control—avirulent ∆icmT mutant bacteria were generated by heat-treatment for 30 h (50°C), 5 h (55°C) or 30 min (60°C) (Figure S2D). The VBNC bacteria were then exposed to A. castellanii, A. polyphaga or D. discoideum for up to 4 weeks (Figure 3A–C). Under the conditions used, co-incubation of VBNC L. pneumophila JR32 with amoebae did not show any resuscitation as defined as intracellular bacterial growth. The lack of resuscitation was also observed upon varying the multiplicity of infection (MOI 1–100), the buffer (Ac buffer, Page's amoeba saline solution) or the incubation temperature of infected A. castellanii (30°C, 37°C). The Ac buffer used for the cultivation of A. castellanii and A. polyphaga restricted the extracellular growth of untreated or heat-treated L. pneumophila for at least 7 days or 4 weeks, respectively (Figure S4A,B), while the MB buffer used for D. discoideum restricted the extracellular bacterial growth for the entire 4 weeks (Figure S4C).
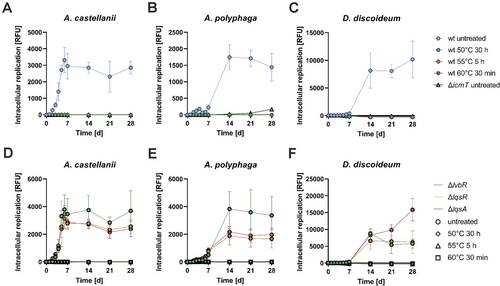
The Lqs-LvbR system regulates the emergence of non-growing L. pneumophila ‘persisters’ in phagocytes (Personnic et al. 2019; Striednig et al. 2021). Accordingly, we sought to assess whether the absence of lvbR, lqsR or lqsA affects the resuscitation of heat-induced VBNC L. pneumophila in amoebae. To this end, GFP-producing VBNC ∆lvbR, ∆lqsR and ∆lqsA mutant bacteria were generated by heat-treatment for 30 h (50°C), 5 h (55°C) or 30 min (60°C) (Figure S2E), and exposed to A. castellanii, A. polyphaga or D. discoideum for up to 4 weeks (Figure 3D–F). However, co-incubation of VBNC ∆lvbR, ∆lqsR or ∆lqsA with amoebae did not show any resuscitation, and the heat-treated strains did also not grow extracellularly in the media (Figure S4D–F). In summary, these results indicate that under the conditions used heat-induced VBNC L. pneumophila do not resuscitate upon contact with amoebae, and the Lqs-LvbR network does not seem to affect the lack of intracellular resuscitation.
2.4 VBNC L. pneumophila Are Impaired for Uptake by Amoebae
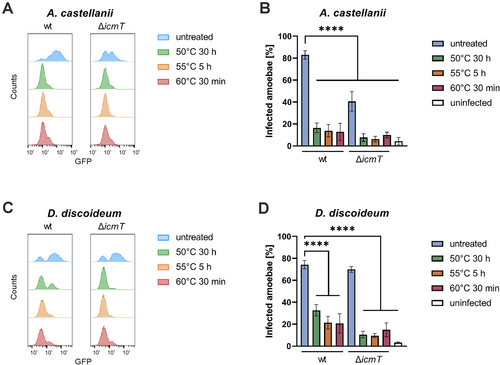
The observed lack of resuscitation of VBNC L. pneumophila by amoebae suggests that heat treatment might impair bacterial virulence. To test this hypothesis and analyse the virulence of heat-induced VBNC L. pneumophila in detail, we examined each infection step individually. The first step, uptake by phagocytic cells, is actively facilitated by the Icm/Dot T4SS (Hilbi, Segal, and Shuman 2001).
The uptake of GFP-producing L. pneumophila by A. castellanii or D. discoideum was assessed by flow cytometry, and amoebae that have taken up GFP-labelled L. pneumophila were distinguished from uninfected cells by bisectional gating (Figure S5A,B). Intracellular untreated or heat-treated VBNC L. pneumophila wild-type (JR32) or Icm/Dot deficient (ΔicmT) bacteria were quantified at 30 min post infection for A. castellanii (Figures 4A,B and S2F) or D. discoideum (Figures 4C,D and S2G). The uptake efficiency of heat-induced VBNC L. pneumophila was approximately 4-fold reduced in both amoeba genera (Figure 4B,D). The uptake reduction of VBNC L. pneumophila JR32 is in agreement with the notion that the function of the Icm/Dot T4SS might be heat-sensitive. However, heat exposure also reduced the uptake of VBNC ΔicmT mutant bacteria by the amoebae, indicating that there are other heat-sensitive factors implicated in the uptake. Taken together, heat-induced VBNC L. pneumophila wild-type as well as ΔicmT mutant bacteria are impaired for uptake by amoebae, suggesting that the function of the Icm/Dot T4SS and/or other bacterial components for bacterial uptake might be heat-sensitive.
2.5 VBNC L. pneumophila Do Not Form LCVs in D. discoideum
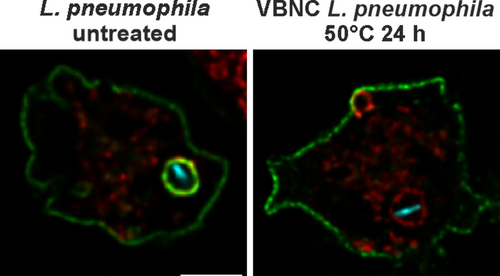
Following uptake, L. pneumophila evades phagolysosomal degradation and establishes a replication-permissive LCV, which is characterised by endosomal markers, the phosphoinositide lipid PtdIns(4)P, and tight association with the ER (Hubber and Roy 2010; Steiner, Weber, and Hilbi 2018; Lockwood et al. 2022). To assess LCV formation by heat-induced VBNC L. pneumophila, we employed confocal microscopy and dually labelled D. discoideum producing the endosomal transporter AmtA-mCherry and the PtdIns(4)P/LCV probe P4C-GFP (Figures 5A and S2H). Upon infection of the amoebae with mCerulean-producing untreated or VBNC L. pneumophila JR32 or ΔicmT mutants, the untreated wild-type bacteria robustly formed LCVs, while no LCVs were observed for VBNC L. pneumophila or the Icm/Dot-defective ΔicmT mutant strain.
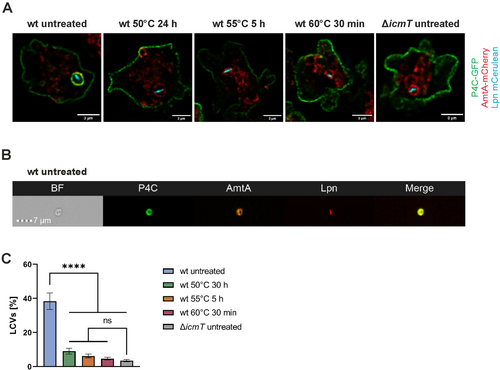
To detect and quantify LCV formation by heat-induced VBNC L. pneumophila in an unbiased manner and at larger numbers, we used imaging flow cytometry (IFC), allowing high-throughput image analysis, albeit at limited spatial resolution. To this end, we assessed homogenates of dually labelled D. discoideum producing AmtA-mCherry and P4C-GFP, infected with mPlum-producing untreated or VBNC L. pneumophila wild-type (JR32) or ΔicmT mutants (Figures 5B and S2I). The quantification of LCV formation by IFC revealed that untreated wild-type L. pneumophila formed LCVs with 40% efficiency (Figure 5C). In contrast, 4–8 times fewer LCVs were detected for heat-induced VBNC L. pneumophila, and the low percentage of LCVs observed for the VBNC bacteria was statistically not significantly different from the LCVs detected in ∆icmT-infected amoebae. Owing to the rather low spatial resolution of IFC, we consider these LCVs false positives. In summary, high-resolution fluorescence microscopy and unbiased, high-throughput IFC data indicate that heat-induced VBNC L. pneumophila do not form LCVs in D. discoideum.
2.6 Heat Exposure Rapidly Inactivates the Icm/Dot T4SS of Culturable L. pneumophila
Given that heat-induced VBNC L. pneumophila is impaired for Icm/Dot-dependent phagocyte uptake (Figure 4) and LCV formation (Figure 5), we sought to assess whether the T4SS is directly affected by the heat treatment. To this end, we tested the effector translocation efficiency of VBNC L. pneumophila with a β-lactamase assay, which has been established for mammalian cells (de Felipe et al. 2008; Charpentier et al. 2009). In this approach, L. pneumophila producing effector proteins fused to a TEM β-lactamase is used to infect host cells, which are loaded with the diffusible β-lactamase substrate CCF4-AM. The translocation efficiency of the effector fusion protein can be quantified via β-lactamase activity within the cells, by measuring the fluorescence of the cleaved (emission at 460 nm) versus the intact (emission at 530 nm) CCF4-AM substrate.
To determine the functionality of the Icm/Dot T4SS, we infected RAW 264.7 macrophages with untreated or heat-treated VBNC L. pneumophila, and the translocation efficiency of the effector LepB-β-lactamase fusion protein was assessed (Figures 6A and S2J). While the fusion protein was efficiently translocated into macrophages by untreated L. pneumophila wild-type (JR32), heat treatment (50°C, 30 h; 55°C, 5 h; or 60°C, 30 min) completely abolished the translocation by these VBNC bacteria. The same lack of translocation was observed for LepB produced by the Icm/Dot-deficient ΔicmT mutant strain and for the housekeeping enzyme FabI produced by strain JR32 (Figure 6A). Next, we assessed whether the decrease in effector translocation efficiency impairs the virulence of L. pneumophila. To this end, RAW 264.7 macrophages were infected with GFP-producing VBNC L. pneumophila (50°C, 30 h; 55°C, 5 h; or 60°C, 30 min), and intracellular replication was monitored over the course of 6 days (Figures 6B and S2K). Upon treatment at 50°C, 55°C or 60°C, the VBNC L. pneumophila were not resuscitated and accordingly, did neither grow in macrophages (Figures 6B and S2K) nor in RPMI 1640 medium alone (Figure S4G). The findings are in full agreement with the reduced effector protein translocation efficiency observed for heat-induced VBNC L. pneumophila.
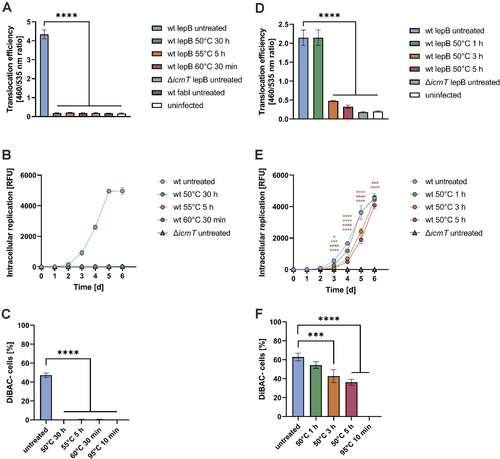
Finally, we assessed the membrane potential of heat-induced VBNC L. pneumophila using DAPI/DiBAC staining—the dye DiBAC primarily enters and stains depolarized cells (Figure S1C). While almost 50% of untreated L. pneumophila were DiBAC-negative, upon heat exposure (50°C, 30 h; 55°C, 5 h; or 60°C, 30 min) all bacteria turned DiBAC-positive, and therefore, their membrane potential was completely abolished (Figures 6C and S2L). Taken together, heat-induced VBNC L. pneumophila are impaired for T4SS-dependent effector translocation into macrophages and intracellular replication, and they lack a measurable membrane potential.
To shed light on the kinetics of Icm/Dot T4SS heat-inactivation, we exposed L. pneumophila to 50°C for 1, 3 or 5 h and assessed effector translocation into RAW 264.7 macrophages. Under these conditions, treatment at 50°C for as short as 3 h significantly reduced the effector translocation efficiency (Figure 6D). The culturability of the bacteria was not compromised upon exposure to 50°C for 1 or 3 h, and only ca. 1 order of magnitude fewer CFU were obtained upon exposure to 50°C for 5 h (Figure S2M). To assess whether the translocation defect is due to an impaired uptake as observed previously for heat-induced VBNC L. pneumophila and amoebae (Figure 4), we tested the uptake of L. pneumophila exposed to 50°C for 1, 3 or 5 h by RAW 264.7 macrophages. Compared to untreated L. pneumophila, no significant uptake reduction of heat-treated bacteria was observed (Figure S6A,B), while the culturability decreased ca. 1–2 orders of magnitude (Figure S6C).
To test whether the decrease in effector translocation efficiency impairs the virulence of L. pneumophila, RAW 264.7 macrophages were infected with GFP-producing L. pneumophila exposed to 50°C for 1, 3 or 5 h, and intracellular replication was monitored over the course of 6 days (Figures 6E and S2N). L. pneumophila exposed to 50°C displayed a delayed intracellular replication, especially the bacteria treated for 3 or 5 h. In contrast, no bacterial growth was observed in RPMI 1640 medium alone (Figure S4H). Finally, we also assessed the membrane potential of heat-treated L. pneumophila using DAPI/DiBAC staining (Figures 6F and S2O). Interestingly, L. pneumophila exposed to 50°C for 1, 3 or 5 h showed a continuously decreasing membrane potential. In summary, experiments aimed at studying the effects of heat on the Icm/Dot T4SS revealed a rapid loss of function of the T4SS by exposure to as low as 50°C for as short as 3 h.
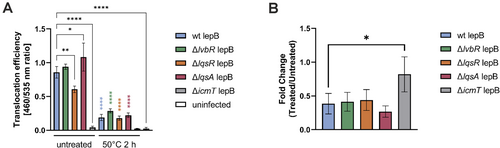
2.7 The Lqs-LvbR System Regulates the Translocation Efficiency but Not Heat Inactivation of the Icm/Dot T4SS
Given the potential heat-protective role of the Lqs-LvbR network indicated by the viability staining (Figure 2), we assessed the effects of heat-treatment on the Icm/Dot T4SS function in the ∆lvbR, ∆lqsR or ∆lqsA mutant strains (Figure 7). The ∆icmT mutant was used as a negative control. Notably, even without heat treatment, the effector translocation efficiency of ∆lqsR and ∆lqsA mutants differed from the parental strain JR32. Specifically, the ∆lqsR mutant exhibited significantly lower translocation efficiency compared to the wild-type strain, whereas the ∆lqsA mutant showed increased translocation (Figure 7A). Upon heat treatment at 50°C for 2 h, all strains showed a significant decrease in translocation efficiency. However, these conditions did not affect bacterial growth (Figure S2P). The extent of the heat-inactivation, expressed as a fold change, revealed that regardless of the initial translocation efficiency, the heat treatment decreased the translocation to a similar degree for the wild-type as well as the Lqs-LvbR mutant strains (Figure 7B). Taken together, these results indicate that the Icm/Dot T4SS is rapidly inactivated by heat treatment, independent of possible heat-protective functions of components of the Lqs-LvbR network. Overall, the results outlined in this study reveal that a rapid temperature-dependent inactivation of the Icm/Dot T4SS impairs the resuscitation of heat-induced VBNC L. pneumophila by phagocytes.
3 Discussion
In this study, we document that L. pneumophila wild-type and ∆icmT mutant bacteria enter the VBNC state after exposure to heat stress (50°C, 30 h; 55°C, 5 h; or 60°C, 30 min), while still retaining metabolic activity and an intact membrane (Figure 1). We did not observe resuscitation of heat-induced VBNC L. pneumophila by different amoebae (Figure 3) or RAW 264.7 macrophages (Figure 6B). Accounting for these observations, the VBNC bacteria showed an impaired virulence in several discrete and well-defined steps during the infection, such as reduced uptake by phagocytes (Figure 4), failure to form LCVs (Figure 5) and diminished translocation of effector proteins through the Icm/Dot T4SS (Figure 6). Furthermore, the L. pneumophila ∆lvbR and ∆lqsR mutant strains were less heat resistant than the parental strain, while the ∆lqsA mutant was more heat resistant (Figure 2), yet the Lqs-LvbR system did not affect the heat inactivation of the Icm/Dot T4SS, and consequently, had no effect on the lack of resuscitation of heat-induced VBNC bacteria by amoebae (Figure 7).
Membrane-bound ‘nanomachines’ of Gram-negative bacteria, such as Type IV and Type III secretion systems and the flagellar apparatus are powered by ATP as well as by electrochemical proton or sodium ion gradients across the inner bacterial membrane (Minamino and Imada 2015). Accordingly, dissipation of the proton motive force (PMF)—that is the electric potential and the proton concentration gradient—by protonophores inhibits these machineries. Thus, the Icm/Dot T4SS of L. pneumophila (Charpentier et al. 2009), Type III secretion systems of Shigella flexneri (Schroeder, Jann, and Hilbi 2007) and Yersinia enterocolitica (Wilharm et al. 2004), as well as flagellar protein export by Salmonella enterica (Minamino and Namba 2008; Paul et al. 2008) and flagellar rotation (Minamino and Imada 2015) have been shown to be inhibited by the protonophore CCCP (carbonyl cyanide m-chlorophenylhydrazone). Our finding that short-term heat treatment (50°C, 3–5 h) diminishes the Icm/Dot-dependent translocation of the effector protein LepB (Figure 6D) and reduces the membrane potential of L. pneumophila (Figure 6F), indicates that the dissipation of the membrane potential (and hence the collapse of the PMF) mechanistically accounts—at least partially—for the heat-induced inhibition of the T4SS. Additionally, the thermal denaturation of Icm/Dot components might also contribute to the functional defect of the T4SS. In agreement with a post-transcriptional/post-translational mechanism of heat-induced inhibition of Icm/Dot-dependent secretion, components of the Lqs-LvbR regulatory network did not affect heat inactivation (Figure 7).
LCV formation was assessed by confocal microscopy and IFC in D. discoideum infected with untreated or VBNC L. pneumophila (Figure 4). The results showed that wild-type heat-induced VBNC L. pneumophila do not form LCVs, similar to avirulent ∆icmT mutant bacteria. These observations are in agreement with the notion that the heat exposure compromises the functionality of the Icm/Dot T4SS, thereby preventing the translocation of the effector proteins necessary for LCV formation. Since LCV formation is a prerequisite for intracellular L. pneumophila replication, the absence of LCVs explains the observed lack of resuscitation and growth. Indeed, heat-induced VBNC L. pneumophila do not replicate in amoebae (Figure 3A–C) or macrophages (Figure 6B). Furthermore, a less harsh heat treatment of L. pneumophila, which is sufficient to impair Icm/Dot-dependent effector translocation but leaves the cells culturable, still results in delayed intracellular replication in the macrophages (Figure 6E). Overall, these findings suggest that the lack of resuscitation of heat-induced VBNC L. pneumophila by amoebae and macrophages is owing to the inactivation of the Icm/Dot T4SS.
In addition to the Icm/Dot-dependent effects of heat stress on L. pneumophila, we also observed effects which are evidently not dependent on the T4SS. Heat-induced VBNC ∆icmT mutant bacteria, which lack a functional Icm/Dot T4SS, were taken up less efficiently by A. castellanii (Figure 4B) and D. discoideum (Figure 4D) compared to the untreated strain. VBNC L. pneumophila thus treated lack a membrane potential (Figure 6C), and therefore, (a) membrane-associated factor(s) other than the Icm/Dot T4SS accounts for the uptake defect observed. An obvious candidate is the PMF-driven flagellar apparatus of L. pneumophila. The L. pneumophila flagellum is regulated by the Lqs system (Schell, Kessler, and Hilbi 2014; Schell et al. 2016) and is indeed a virulence factor (Schell, Simon, and Hilbi 2016; Appelt and Heuner 2017). L. pneumophila strains lacking the flagellar sigma factor, FliA, or the major flagellum subunit, FlaA, are non-motile and impaired for the invasion of A. castellanii, human HL-60 macrophage-like cells, and mouse bone marrow-derived macrophages (Dietrich et al. 2001; Molofsky, Shetron-Rama, and Swanson 2005). Moreover, compared to biofilms formed by L. pneumophila JR32, A. castellanii amoebae migrated more slowly through biofilms formed by ∆flaA mutant bacteria, and the ∆flaA mutants did not form clusters on the surface of the amoebae (Hochstrasser et al. 2022), indicating that the flagellum is a major determinant of pathogen-amoeba interactions. At this point, we cannot rule out that the heat-induced denaturation of L. pneumophila virulence factors other than the Icm/Dot T4SS or the flagellum contribute to the reduced uptake observed.
The effector protein translocation efficiencies of untreated Lqs-LvbR mutant strains were different from the parental strain but not affected by heat treatment (Figure 7). The reduced translocation efficiency of the ∆lqsR mutant, lacking the response regulator LqsR, correlates with the observation that LqsR inhibits entry into the replicative, non-virulent growth phase and promotes virulence (Tiaden et al. 2007, 2008). On the other hand, the finding of an increase in translocation of the ∆lqsA mutant does not match the observation that an overexpression of lqsA promotes intracellular replication (Fan et al. 2023). However, increased intracellular growth was only observed upon overexpression of lqsA under a strong promoter and not upon expression under the control of its endogenous PlqsA promoter. Moreover, the unchanged translocation efficiency of the ∆lvbR mutant, lacking the pleiotropic transcription factor LvbR, does not correlate with the impaired virulence phenotype of the mutant (Hochstrasser et al. 2019). Taken together, these results suggest that there is no direct and straightforward correlation between the overall efficiency of effector protein translocation and the virulence phenotype of L. pneumophila regulatory mutants.
Compared to the parental strain JR32, the ∆lvbR and ∆lqsA mutant strains show a reduced cell density at 45°C, and therefore, LvbR and LqsA regulate bacterial growth at higher temperatures (Hochstrasser and Hilbi 2022). In this study, the ∆lvbR and ∆lqsA mutant strains show an increased (∆lvbR) or decreased (∆lqsA) number of dead cells at temperatures beyond 50°C (Figure 2). Accordingly, there is a correlation between growth and survival at higher temperatures for LvbR but not for LqsA. However, both LvbR and LqsA are implicated in the response of L. pneumophila to temperatures from 45°C to 60°C.
The Lqs system and LvbR also negatively regulate the ratio of non-growing versus growing sessile bacteria and positively regulate the frequency of growth resumption of individual L. pneumophila bacteria and microcolony formation (Personnic, Striednig, and Hilbi 2021). Based on these findings, we tested whether the Lqs-LvbR network plays a role in the resuscitation of VBNC L. pneumophila exposed to amoebae. However, under the conditions used, neither heat-induced VBNC L. pneumophila JR32 nor the corresponding ∆lvbR, ∆lqsR, or ∆lqsA mutant strains were resuscitated by A. castellanii, A. polyphaga or D. discoideum (Figure 3). These results are in agreement with the notion that antibiotics-resistant persisters are physiologically distinct from the heat-induced ‘dormant’ VBNC form. In contrast to L. pneumophila, quorum sensing apparently plays a role in VBNC resuscitation of other bacterial species (Li et al. 2014; Pinto, Santos, and Chambel 2015), including Vibrio vulnificus (Ayrapetyan, Williams, and Oliver 2014) and Salmonella enterica (Reissbrodt et al. 2002).
VBNC L. pneumophila induced by starvation, exposure to disinfection agents, or heat treatment were reported to be resuscitated by A. castellanii (Steinert et al. 1997; Alleron et al. 2008; Al-Bana, Haddad, and Garduno 2014; Ducret, Chabalier, and Dukan 2014; Dietersdorfer et al. 2018; Cervero-Arago et al. 2019) or A. polyphaga (Garcia et al. 2007; Epalle et al. 2015; Nisar et al. 2023). However, the resuscitation of VBNC L. pneumophila largely depended on the bacterial strain used (Alleron et al. 2008, 2013), and was heavily influenced by the heat exposure settings and the subsequent experimental conditions (Allegra et al. 2008; Epalle et al. 2015). Moreover, intracellular growth in amoebae and macrophages was observed after heat-induced formation of VBNC L. pneumophila (55°C, 3–8 h; 60°C, 30–60 min) in one study (Cervero-Arago et al. 2019), but culturability of the bacteria was never restored, suggesting that persistence rather than resuscitation was documented. Accordingly, while we were able to robustly and reproducibly induce VBNC formation of L. pneumophila strain JR32 by heat exposure, our failure to resuscitate the bacteria might be due to the specific bacterial and/or amoeba strains used, or due to the precise and carefully controlled experimental conditions applied. It is critical to generate a homogeneous, uniform VBNC population prior to engaging in experimental resuscitation attempts.
Under our conditions, heat-treated VBNC L. pneumophila were not resuscitated by RAW 264.7 macrophages (Figure 6B). In other studies, VBNC L. pneumophila were found to be resuscitated by A. castellanii but not in a guinea pig animal model (Steinert et al. 1997), by A. polyphaga but not in differentiated human macrophage-like cells (U937, HL-60) or A549 alveolar cells (Epalle et al. 2015), or by A. castellanii as well as by primary human monocyte-derived macrophages (Dietersdorfer et al. 2018). Accordingly, the specific host cells used, the distinct L. pneumophila strain, and the distinct experimental conditions appear to determine the outcome of resuscitation attempts. Future studies will address in more detail the important question of which host cell factors determine the resuscitation of VBNC L. pneumophila.
In summary, our results are in agreement with the notion that already a rather mild and brief heat treatment of L. pneumophila leads to the dissipation of the PMF/membrane potential and concomitantly impairs the function of membrane-bound virulence machineries, such as the Icm/Dot T4SS. Heat-induced VBNC L. pneumophila did not resuscitate in co-culture with amoebae or macrophages under the conditions used, despite retaining metabolic activity and membrane integrity. Future studies will aim at investigating the possible recovery of the membrane potential and the mechanism of resuscitation of VBNC L. pneumophila either by amoebae or macrophages or under cell-free conditions.
4 Experimental Procedures
4.1 Bacteria, Eukaryotic Cells, and Growth Conditions
L. pneumophila strains (Table 1) were grown on CYE agar plates (Feeley et al. 1979) at 30°C for 5 days followed by liquid cultures in N-(2-acetamido)-2-aminoethanesulfonic acid (ACES; AppliChem, Darmstadt, Germany)-buffered yeast extract (AYE) medium (Horwitz 1983) for 24 h on a wheel (80 rpm). AYE medium was supplemented with chloramphenicol (Cm, 5 μg/mL; Roche, Mannheim, Germany) to maintain plasmids and with 1 mM IPTG (Roth, Germany) to induce gene expression if required.
| Strain or plasmid | Relevant properties | Reference |
|---|---|---|
| L. pneumophila | ||
| JR32 | L. pneumophila Philadelphia-1, serogroup 1, salt-sensitive isolate of AM511 | Sadosky, Wiater, and Shuman (1993) |
| AK03 (ΔlvbR) | JR32 lvbR::Km | Hochstrasser et al. (2019) |
| GS3011 (ΔicmT) | JR32 icmT3011::Km | Segal, Purcell, and Shuman (1998) |
| NT02 (ΔlqsA) | JR32 lqsA::Km | Tiaden et al. (2010) |
| NT03 (ΔlqsR) | JR32 lqsR::Km | Tiaden et al. (2007) |
| Plasmids | ||
| pAW014 | pMMB207C, ΔlacIq, Ptac-mPlum (constitutive), Cm | Steiner et al. (2017) |
| pNP99 | pMMB207C, ΔlacIq, Ptac-mCerulean (constitutive), Cm | Steiner et al. (2017) |
| pNP102 | pMMB207C, ΔlacIq, Ptac-mCherry (constitutive), Cm | Steiner et al. (2017) |
| pNT28 | pMMB207C, ΔlacIq, Ptac-gfp (constitutive), Cm | Tiaden et al. (2007) |
| pXDC61-FabI | pMMB207C, blaM-fabI, Cm | de Felipe et al. (2008) |
| pXDC61-LepB | pMMB207C, blaM-lepB, Cm | de Felipe et al. (2008) |
- Abbreviations: Cm, chloramphenicol resistance; Km, kanamycin resistance.
A. castellanii (ATCC 30234, laboratory collection) and A. polyphaga (ATCC 50362, laboratory collection) (Conza, Casati, and Gaia 2013) were cultured in proteose-yeast extract-glucose (PYG) medium (Moffat and Tompkins 1992) at 23°C using Bacto proteose peptone (Life Technologies, Paisley, UK) and Bacto yeast extract (Life Technologies, USA). D. discoideum (Ax3, laboratory collection) were grown in HL5 medium (ForMedium, Hunstanton, UK) at 23°C and—where indicated—transfected by electroporation with a Gene Pulser Xcell (Bio-Rad, Cressier, Switzerland) device as described (Weber, Wagner, and Hilbi 2014). After 24 h, transfectants were selected and maintained in HL5 medium containing hygromycin (50 μg/mL; Invitrogen, Carlsbad, CA, USA) and/or geneticin (G418, 20 μg/mL; Roth, Germany). Murine RAW 264.7 macrophages (ATCC TIB-71, laboratory collection) were grown in RPMI 1640 medium (Life Technologies, UK) supplemented with 10% fetal calf serum (FCS; Life Technologies, UK) and 1% L-glutamine (Life Technologies, UK) in a humidified atmosphere at 37°C in 5% CO2.
All chemicals were from Sigma-Aldrich (Steinheim, Germany) or Roth (Karlsruhe, Germany), unless stated otherwise.
4.2 Heat Treatment of L. pneumophila
L. pneumophila strains were grown in AYE media for 24 h at 37°C to stationary phase. The bacteria were washed once and subsequently diluted to an OD600 of ca. 0.1 (~2 × 108 bacteria/ml) in 10 mL ACES-buffered H2O (pH 6.9 ± 0.1) in a 50 mL Falcon tube. ACES-buffered H2O was supplemented with Cm (5 μg/mL) to maintain plasmids and with IPTG (1 mM) to induce gene expression if required. The bacteria were then exposed to 50°C, 55°C or 60°C in stirred water baths. Untreated bacteria were left at room temperature (RT). Heat-treated and untreated L. pneumophila were assessed in each experiment for their culturability by dilution plating.
4.3 Flow-Cytometry of Heat-Treated L. pneumophila Using Fluorescent Dyes and Proteins
Fluorescein diacetate (FDA; Sigma-Aldrich) was used for staining and estimation of metabolic activity, and the Live-or-Dye 640/662 stain (LD, #32007; Biotium, Fremont, CA, USA) was used to assess membrane integrity. Five hundred-microliter heat-treated, untreated and heat-inactivated (95°C, 10 min) L. pneumophila previously adjusted to an OD600 of 0.1 (ca. 2 × 108 bacteria/ml) were collected (ca. 2000 × g, 5 min) and washed once with Dulbecco's phosphate buffered solution (DPBS; Life Technologies, UK). The bacteria were then simultaneously stained with the two viability dyes by resuspension in 900 μL LD staining solution (1:1000 LD in DPBS) and 100 μL FDA staining solution (50 μg/mL FDA in acetone). Sample tubes were vortexed and incubated for 45 min at RT in the dark. After washing with DPBS, the bacteria were fixed with 4% paraformaldehyde (PFA; Electron Microscopy Sciences, Hatfield, England) for 30 min at RT in the dark. Then, the bacteria were counterstained in 300 μL 4′,6-diamidin-2-phenylindol (DAPI; Roth, Karlsruhe, Germany) staining solution (1 μg/mL DAPI in DPBS) for 30 min at RT in the dark. The bacteria were washed once with DPBS and directly analysed by FACS.
The BD Cell Viability kit (#349483; BD Biosciences, Allschwil, Switzerland) was used for staining and quantitation of alive, dead and intermediate cell populations. Five hundred-microliter heat-treated, untreated and heat-inactivated (95°C, 10 min) L. pneumophila, previously adjusted to an OD600 of 0.1 (ca. 2 × 108 bacteria/mL) were collected (ca. 2000 × g, 5 min) and resuspended in 500 μL filter-sterilised staining buffer (1 mM EDTA (AppliChem, Darmstadt, Germany) and 0.01% Tween-20 (Roth, Karlsruhe, Germany) in DPBS, pH 7.4 ± 0.1) containing 420 nM of thiazole orange (TO) and 48 μM propidium iodide (PI). Sample tubes were vortexed and incubated for 15 min at RT in the dark, then put on ice and directly analysed by FACS.
DiBAC4(5) [Bis-(1,3-dibutylbarbituric acid)pentamethine oxonol] (DiBAC; AAT Bioquest, Sunnyvale, CA, USA), a fluorescent dye that enters primarily depolarized cells, was used to monitor changes in membrane potential. Five hundred-microliter heat-treated, untreated and heat-inactivated (95°C, 10 min) L. pneumophila previously adjusted to an OD600 of 0.1 (ca. 2 × 108 bacteria/ml) were collected (ca. 2000 × g, 5 min), washed once with DPBS, and stained by resuspension in 500 μL DiBAC staining solution (10 μM DiBAC in DPBS). Sample tubes were vortexed and incubated for 10 min at RT in the dark. After washing with DPBS, the bacteria were counterstained in 300 μL DAPI staining solution (1 μg/mL DAPI in DPBS) for 30 min at RT in the dark. The bacteria were washed once with DPBS and directly analysed by FACS.
To assess the stability of heat-treated GFP, mCherry and mPlum fluorescent proteins in L. pneumophila flow cytometry was used. Briefly, 500 μL heat-treated (60°C, 30 min), untreated and heat-inactivated (95°C, 10 min) fluorescent protein-producing L. pneumophila, previously adjusted to an OD600 of 0.1 (ca. 2 × 108 bacteria/ml) were collected (ca. 2000 × g, 5 min) and washed once with DPBS. The bacteria were fixed with 4% PFA for 30 min at RT in the dark, counterstained in 300 μL DAPI staining solution (1 μg/mL DAPI in DPBS) for 30 min at RT in the dark and washed twice in DPBS.
FACS analysis was performed using a Fortessa II flow cytometer and Diva software. The bacterial populations were identified by forward (FSC, 650 V) and sideward scatter (SSC, 300 V) gating, with a threshold of 200 each, and 10,000 events per sample were recorded. The samples were further assessed using the DAPI signal (Vio 450_30, 700 V), where indicated. Some samples were examined for FDA (Blue 530_30, 700 V) and LD (Red 670_14, 700 V) signals, for TO (Blue 530_30, 700 V) and PI (YG 610_20, 700 V), for DAPI and DiBAC (YG 610_20, 700 V), or for DAPI and GFP (Blue 530_30, 700 V), mCherry (YG 610_20, 700 V) or mPlum (YG 670_30, 700 V) signals.
The data were analysed with the software FlowJo. The FDA-positive and LD-negative cell population was gated using the untreated, the heat-inactivated and the unstained samples as reference to position the quadrant gate. For TO/PI staining, alive, dead and intermediate cell populations were gated using the untreated and heat-inactivated samples as reference to position the quadrant gate, and a blank control of staining buffer containing dyes was used to confirm that debris were not being stained with the dyes and could be excluded in the gated populations. For DAPI/DiBAC staining, the DiBAC-negative cell population was gated using the untreated and the heat-inactivated populations as reference to set a gate, as well as a control population of only DAPI stained bacteria. Finally, for fluorescent protein stability, a population of only DAPI stained bacteria without a fluorophore-producing plasmid was used to gate the populations.
4.4 Analysis of Phagocytosis by Flow Cytometry
Phagocytosis of L. pneumophila by A. castellanii, D. discoideum or RAW 264.7 macrophages was analysed as published (Weber et al. 2006) by flow cytometry using GFP-producing bacteria. Briefly, the amoebae or macrophages were seeded in PYG, HL5 or RPMI, respectively, in 6-well plates (1 × 106 cells/well) and left to adhere at 23°C (amoebae) or 37°C in 5% CO2 (macrophages) for 24 h (cells approximately doubled in this time). Heat-treated or untreated L. pneumophila producing GFP were diluted in PYG, HL5 or RPMI to the desired density and used to infect the amoebae (MOI 10) or the macrophages (MOI 100). The infections were synchronised by centrifugation (500 × g, 10 min, RT) and incubated at 30°C (A. castellanii), 25°C (D. discoideum) or 37°C in 5% CO2 (RAW 264.7 macrophages). Thirty-minute post-infection, extracellular bacteria were removed by washing three times with PYG, HL5 or prewarmed DPBS. Infected host cells were detached by vigorously pipetting and fixed with 4% PFA for 30 min at RT in the dark, washed once with DPBS and directly analysed by FACS.
The GFP-positive amoebae or macrophage population was quantified using a Fortessa II flow cytometer and Diva software. The host cell population was identified employing forward (FSC, 250 V) and sideward scatter (SSC, 225 V) gating, with a threshold of 200 each, and examined for the GFP signal (Blue 530_30, 500 V). 10,000 events per sample were recorded. The data were analysed with the software FlowJo. The GFP-positive host cell population was gated using an uninfected control as reference.
4.5 Confocal Microscopy of L. pneumophila-Infected, Dually Labelled D. discoideum
LCV formation of L. pneumophila in D. discoideum was analysed by confocal microscopy using mCerulean-producing bacteria, adapted from Vormittag, Hüsler, et al. (2023). Briefly, dually labelled D. discoideum amoebae producing AmtA-mCherry (endosomal marker) and P4C-GFP (LCV marker) were seeded in 6-well plates (1 × 106 cells/well) containing HL5 medium, geneticin and hygromycin, and left to adhere at 23°C for 24 h (cells approximately doubled in this time). Heat-treated or untreated L. pneumophila producing mCerulean were washed once, diluted in HL5 to the desired density and used to infect the amoebae at an MOI of 100 or an MOI of 10, respectively. The infection was synchronised by centrifugation (500 × g, 10 min, RT) and incubated at 25°C. Two-hour post-infection, extracellular bacteria were removed by washing three times.
For visualisation by confocal fluorescence microscopy (Leica SP8 inverse, 63× oil objective), the infected amoebae were collected and fixed with 4% PFA (30 min), washed once with DPBS, transferred to an 18-well μ-slide dish (Ibidi) and immobilised by adding a layer of DPBS/0.5% agarose (Personnic, Striednig, and Hilbi 2019). Images were deconvolved with Huygens professional software version 19.10 (Scientific Volume Imaging; http://svi.nl) using the CMLE algorithm with a maximum of 94 iterations and a quality change threshold of 0.02, set to an SNR of 10.0. Images were finalised/exported using the ImageJ software version 2.14.0.
4.6 Imaging Flow Cytometry of Phagosomes in Lysates of Infected D. discoideum
LCV formation of L. pneumophila in D. discoideum was analysed by IFC using mPlum-producing bacteria, adapted from Hüsler et al. (2021). Briefly, dually labelled D. discoideum amoebae producing AmtA-mCherry (endosomal marker) and P4C-GFP (LCV marker) were seeded in 6-well plates (1 × 106 cells/well) containing HL5 medium, geneticin and hygromycin, and left to adhere at 23°C for 24 h (cells approximately doubled in this time). Heat-treated or untreated L. pneumophila producing mPlum were diluted in HL5 to the desired density and used to infect the amoebae at an MOI of 100. The infection was synchronised by centrifugation (500 × g, 10 min, RT) and incubated at 25°C. Two-hour post-infection, extracellular bacteria were removed by washing twice.
Infected amoebae were detached by vigorously pipetting and washed once with ice cold SorC buffer (2 mM Na2HPO4, 15 mM KH2PO4, 50 μM CaCl2 × 2 H2O, pH 6.0), before resuspending in ice cold homogenization buffer (20 mM HEPES, 250 mM sucrose, 0.5 mM EGTA, pH 7.2) containing a protease inhibitor cocktail tablet (Roche, Mannheim, Germany). Cells were homogenised using a ball homogeniser (Isobiotec, Heidelberg, Germany) with an exclusion size of 8 μm. The lysate was fixed with 4% cold PFA on ice for 30 min and then washed once with DPBS and resuspended in 20 μL ice cold DPBS prior to IFC analysis.
Using an imaging flow cytometer (ImageStreamX MkII, Amnis), 100,000 events were recorded and analysed with IDEAS software version 6.2 (Amnis). Phagosomes containing one L. pneumophila bacteria were gated by assessing the size and the colocalization of AmtA-mCherry with the mPlum signal produced by the bacteria. LCVs were identified by the colocalization of phagosomes with P4C-GFP.
4.7 Intracellular Replication of L. pneumophila in Amoebae and Macrophages
Intracellular replication of L. pneumophila in A. castellanii, A. polyphaga, D. discoideum and RAW 264.7 macrophages was determined as published (Hochstrasser et al. 2019). Briefly, the host cells were seeded in PYG, HL5 or RPMI, respectively, in black clear bottom 96-well plates (2 × 104 cells/well) and left to adhere at 23°C (amoebae) or 37°C in 5% CO2 (macrophages) for 24 h (cells approximately doubled in this time). Heat-treated or untreated L. pneumophila producing GFP were diluted in Ac buffer (A. castellanii, A. polyphaga) (Moffat and Tompkins 1992), MB (D. discoideum) (Solomon et al. 2000) or RPMI (macrophages) to the desired density and used to infect the host cells at an MOI of 1. The infection was synchronised by centrifugation (500 × g, 10 min, RT) and incubated at 30°C (A. castellanii, A. polyphaga), 25°C (D. discoideum), or 37°C in 5% CO2 (macrophages).
Intracellular bacterial replication was assessed by measuring GFP production with a plate reader. Possible extracellular replication was monitored in wells containing only heat-treated or untreated L. pneumophila in Ac buffer, MB or RPMI without host cells. To counteract evaporation in experiments lasting up to 4 weeks, the outermost wells were filled with H2O and refilled with fresh H2O at 14 days post infection.
4.8 Effector Protein Translocation Assay
Icm/Dot-dependent translocation into host cells was determined with a β-lactamase translocation assay, adapted from (Charpentier et al. 2009; Rothmeier et al. 2013). Briefly, RAW 264.7 macrophages were seeded in RPMI in black clear bottom 96-well plates (4 × 105 cells/mL in a final volume of 100 μL/well) and incubated at 37°C in 5% CO2 overnight. Heat-treated or untreated L. pneumophila, producing TEM β-lactamase fusion proteins, were diluted in RPMI to the desired density and used to infect the macrophages at an MOI of 100. The infection was synchronised by centrifugation (500 × g, 10 min, RT) and incubated at 37°C in 5% CO2 for 50 min.
The cells were then loaded with the fluorescent β-lactamase substrate CCF4-AM by adding 20 μL of 6× concentrated solution (LiveBLAzer-FRET B/G loading kit; Invitrogen, USA) containing 3.75 mM probenecid (Sigma-Aldrich, Germany). After 90 min of incubation at RT in the dark, the fluorescence was measured with a fluorescence plate reader (Cytation 5, Agilent BioTek) using an excitation wavelength of 405 nm and an emission of 460 and 535 nm.
The Icm/Dot substrate LepB fused to the TEM β-lactamase in L. pneumophila JR32 served as a positive control, while the same fusion protein in the avirulent ∆icmT mutant lacking a functional Icm/Dot T4SS served as a negative control. To test for non-specific release, the translocation of the cytoplasmic protein FabI fused to the TEM β-lactamase was also assessed. Translocation efficiency was expressed as the emission ratio at 460/535 nm after subtracting blank values (RPMI only), or as the fold change between heat-treated and untreated L. pneumophila.
4.9 Statistics
Statistics were determined by Student's t-tests and one-way or two-way ANOVAs on the means and standard deviations of three replicates. The statistical analysis was performed using the GraphPad Prism software (Version 9.5.1), and differences were deemed statistically significant when the p value was less than 0.05.
Author Contributions
Camille Schmid: investigation, writing – original draft, methodology, visualization, formal analysis, validation. Hubert Hilbi: conceptualization, funding acquisition, writing – original draft, project administration, formal analysis, supervision.
Acknowledgements
We would like to thank Valeria Gaia, EOLAB Bellinzona, for providing A. polyphaga amoebae. We thank Arnd Gildemeister and Barbara Borer (Geberit International AG) for stimulating discussions and feedback. The project was funded by Geberit International AG. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All data are contained in the manuscript.