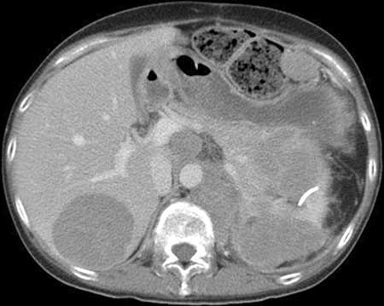
Summary
Definition
History and exam
Key diagnostic factors
- presence of risk factors
- facial plethora
- supraclavicular fullness
- violaceous striae
- absence of pregnancy
- menstrual irregularities
- absence of malnutrition
- absence of alcoholism
- absence of physiological stress
- linear growth deceleration in children
Other diagnostic factors
- female sex
- hypertension
- glucose intolerance or diabetes mellitus
- premature osteoporosis or unexplained fractures
- weight gain and central obesity
- acne
- psychiatric symptoms
- decreased libido
- easy bruisability
- weakness
- facial rounding
- dorsocervical fat pads
- unexplained nephrolithiasis
- venothrombolic event
- hirsutism
Risk factors
- exogenous corticosteroid use
- pituitary adenoma
- adrenal adenoma
- adrenal carcinoma
- neuroendocrine tumours
- thoracic or bronchogenic carcinoma
Diagnostic investigations
1st investigations to order
- urine pregnancy test
- serum glucose
- late-night salivary cortisol
- 1 mg overnight dexamethasone suppression test
- 24-hour urinary free cortisol
- 48-hour 2 mg (low-dose) dexamethasone suppression test
Investigations to consider
- plasma dehydroepiandrosterone sulphate (DHEAS) level
- morning plasma adrenocorticotrophic hormone (ACTH)
- pituitary MRI
- adrenal CT
- high-dose dexamethasone suppression test
- inferior petrosal sinus sampling (IPSS)
- CT of chest, abdomen, and pelvis
- MRI chest
- octreotide scanning
- gallium-68 DOTATATE PET/CT
Treatment algorithm
Contributors
Authors
Maria Fleseriu, MD, FACE
Professor of Medicine (Endocrinology) and Neurological Surgery
Director
Pituitary Center
Oregon Health & Science University
Portland
OR
Disclosures
MF is on the Pituitary Society's Board of Directors. She holds a research grant to the University for Clinical Studies as Principal Investigator for Recordati and Strongbridge, and is an occasional Scientific Consultant for Recordati, HRA Pharma, and Sparrow. MF is an author of several references cited in this topic.
Acknowledgements
Dr Maria Fleseriu would like to gratefully acknowledge Dr Ty Carroll and Dr James Findling, previous contributors to this topic.
Disclosures
TC is an author of a number of references cited in this topic. He is an investigator in clinical trials sponsored by Corcept. JF is an author of a number of references cited in this topic. He is a consultant for, and investigator in, clinical trials sponsored by Corcept and Novartis.
Peer reviewers
Paul M. Stewart, FRCP FMedSci
Professor of Medicine
Director of Research
College of Medical and Dental Sciences
University of Birmingham
Honorary Consultant Physician
Queen Elizabeth Hospital
Birmingham
UK
Disclosures
PMS declares that he has no competing interests.
Antoine Tabarin, MD
Head
Department of Endocrinology
University Hospital of Bordeaux
Pessac
France
Disclosures
AT declares that he has no competing interests.
Liliana Contrersas, MD
Chief
Endocrine Research Department
Instituto de Investigaciones Médicas A. Lanari
University of Buenos Aires and IDIM-CONICET
Buenos Aires
Argentina
Disclosures
LC declares that she has no competing interests.
Philip R. Orlander, MD
Professor of Medicine
Director
Division of Endocrinology, Diabetes & Metabolism
University of Texas Medical School
Houston
TX
Disclosures
PRO declares that he has no competing interests.
Mouhammed Amir Habra, MD, FACP, FACE
Assistant Professor
Department of Endocrine Neoplasia and Hormonal Disorders
Division of Internal Medicine
University of Texas MD Anderson Cancer Center
Houston
TX
Disclosures
MAH declares that he has no competing interests.
Use of this content is subject to our disclaimer