Approach
Diagnosis is based on clinical suspicion and confirmed by serological testing combined with polymerase chain reaction (PCR) or viral culture. Early suspicion, recognition, and management of potential exposure, and rapid identification and treatment of B virus infection are critical to the prevention and management of human disease. An immediate evaluation of the patient should focus on a detailed history of exposure risks, a physical examination targeted to areas relevant to the exposure history (including a neurological examination), and an evaluation for the presence of any skin lesions.
First aid and initial evaluation
If a potential B virus exposure occurs, the critical first step is immediate and thorough cleansing of the exposed area to prevent exposure from progressing to infection. The Centers for Disease Control and Prevention (CDC) has published guidance for wound cleansing.[42]
Immediate and thorough cleansing of the wound or other exposed areas should not be delayed to seek medical care. Although potential exposures are frequent and human infections are rare, each potential exposure should be treated as an emergency until the wound or other exposed areas have been thoroughly cleansed. The exposed area should be cleansed on presentation for medical evaluation regardless of whether or not it was previously cleansed. The exposed area should be thoroughly washed and gently scrubbed with soap, povidone-iodine, detergent, or chlorhexidine and water for 15 minutes, followed by irrigating the area with running water for 15 to 20 minutes. Mucosal exposure (nose, eyes, mouth) should be flushed with sterile saline solution or water for 15 minutes. Specimens should not be collected before washing the area as it could force virus more deeply into the wound.[42]
For those exposed while working with macaque monkeys, for example in a laboratory or zoo, the occupational healthcare provider should be contacted immediately after cleansing the exposed area. The occupational healthcare provider should provide an initial evaluation, assistance with first aid, counselling, and education about the signs and symptoms of B virus infection. They should also facilitate rapid access to an infectious disease specialist with knowledge of B virus.
The diagram below provides an overview of the initial steps that should be carried out following B virus exposure:[Figure caption and citation for the preceding image starts]: Overview of the steps for managing putative B virus exposuresCDC [Citation ends].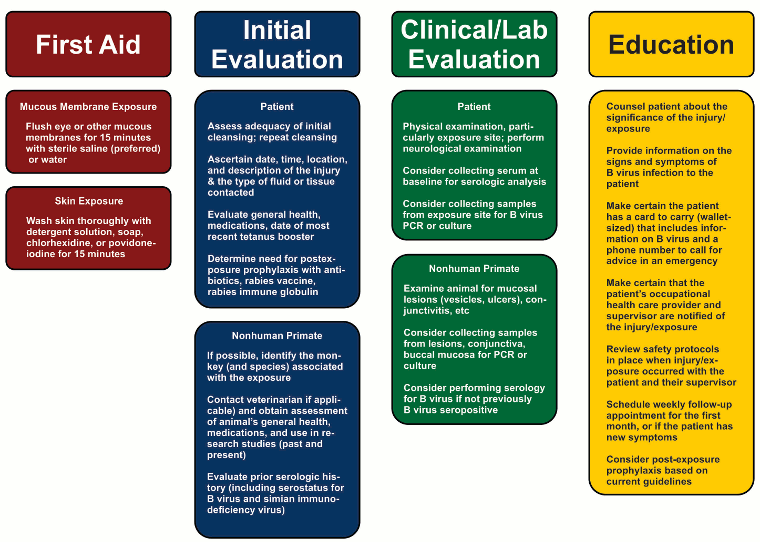
History
A detailed history should be carried out to identify the following:[4]
Date, time, and (geographical) location of exposure
Source of exposure (e.g., macaque monkeys [which includes rhesus, cynomolgus, and other macaque monkeys], body secretions or tissues from macaque monkeys, laboratory cell lines or necropsy material from macaque monkeys, objects contaminated with body fluids or tissues from a macaque monkey). If the source of exposure is an animal, the general health of the animal should be ascertained
Type of exposure (e.g., scratch, bite, percutaneous injury contaminated with macaque body fluids, or mucous membrane or non-intact skin contact with infected body fluid or tissue without injury) and site of infection (on the body)
Cleansing of wound performed following exposure, including how soon after the exposure, if done
Safety protocol in place at the time of exposure
General health, medications taken, and date of most recent tetanus booster.
Identifying the source of exposure
Macaque monkeys (family: Cercopithecidae; subfamily: Cercopithecinae; genus: Macaca) are the only natural hosts for B virus; therefore, identifying the type of monkey responsible for a suspected exposure is important. Non-macaque monkeys (all New World monkeys, and all other Old World monkeys) pose no risk for B virus infection unless they have been co-housed with macaques. If they have been co-housed at any time, latent B virus infection of non-macaques cannot be ruled out.[15][16]
For potential exposures to monkeys that are free-roaming, such as in parks and temples in Asia, it will usually be impossible to determine the type of monkey responsible for the bite or scratch; however, most monkeys in Asia are macaques. Only one species of macaque is native to North Africa (Algeria and Morocco) and Europe (Gibraltar), the Barbary macaque (Macaca sylvanus). Monkeys responsible for bites in Asia should be presumed to be macaques in the absence of any other descriptive evidence. Free-roaming monkeys in Central or South America can generally be presumed not to be macaques. Macaques are not endemic to the western hemisphere, although colonies of imported animals in parts of the US (including animals that are free-roaming) may pose an infection risk to visitors. Such colonies could also exist elsewhere.
Ascertaining general health of the animal responsible
If possible, the medical record of the animal responsible for the exposure should be ascertained to identify its current health status. The medical record should include any history of exposure to infectious agents, and any appearance of active lesions compatible with B virus infection. These mucosal lesions resemble those observed in people infected with HSV-1 or HSV-2. If exposure was in a laboratory setting, the type of research the animal was involved in should be identified where possible.
B virus infection in macaques is usually asymptomatic or only mildly symptomatic, and infected macaques shed virus only intermittently. However, it is important to remember that, while the presence of herpetic skin lesions implies active shedding, B virus-infected macaques with no visible mucosal lesions can still shed virus, similar to humans infected with HSV-1 or HSV-2. As with most mammalian herpesviruses, active shedding of the virus is periodic and, in the case of B virus and closely related alphaherpesviruses (e.g., HSV), may or may not be accompanied by symptoms.[43] Macaques with B virus infection can shed the virus from mucosa (e.g., conjunctiva, buccal) and/or genital surfaces. Macaques appearing unwell, that have been immunologically suppressed (e.g., for research purposes), or have been recently stressed are even more likely to be shedding B virus. B virus can also be deposited on surfaces; therefore, infection of humans and other animals can occur via fomites. Any scratch, bite, percutaneous injury contaminated with macaque body fluids, or mucous membrane or non-intact skin contact with infected fluid from macaques should be regarded as a potential exposure.
Physical examination
A physical examination of the patient should be performed on presentation immediately after cleansing of the exposure site. In the immediate aftermath of exposure, the goal remains to prevent exposure from progressing to infection. Physical examination is aimed at identifying, documenting, and thoroughly cleansing all potentially exposed areas. If the patient presents days after exposure, thorough cleansing of exposed areas should be performed, but is less likely to be of benefit than if performed immediately after exposure.
The type of exposure, appearance of the exposed area, and site of exposure should be carefully examined, but precise risk stratification is discouraged because the limited number of known human B virus disease cases makes it impossible to accurately quantify relative risk of an exposure. Cases of fatal B virus infection in people who could recall no obvious high-risk exposure to macaques or their fluids and tissues have occurred, as have cases among people with exposures that are considered to carry a low risk. The site of infection on the body may, however, impact how the disease progresses. By analogy with rabies, which also spreads by retrograde transport along neural pathways, B virus entry through the head and neck may allow more rapid access to the central nervous system (CNS), where neurological symptoms may precede, or at best coincide with, other symptoms.
A medical examination is focused on gathering evidence of infection. Patients with B virus infection may present with the following:
Signs/symptoms localised to the site of exposure (e.g., herpetic lesions) that worsen or spread to additional locations
Signs/symptoms limited to the peripheral nervous system or the CNS
Systemic flu-like illness (e.g., fever, chills, general malaise, headache, or myalgia) without localising symptoms.
Those with potential exposure should, therefore, be evaluated for the presence of any skin lesions, fever, or neurological symptoms, as these may suggest B virus infection. All presentations can progress to abrupt onset of CNS symptoms. A neurological examination to establish a baseline status and, if the patient is symptomatic, appropriate clinical evaluation of the CNS (e.g., lumbar puncture, brain MRI, EEG) should be carried out to identify and characterise infection and to further characterise the neurological status of the patient.
The history and physical/medical examination should be considered before deciding on whether to administer post-exposure prophylaxis (PEP).[44] Prophylaxis is most likely to be effective in the immediate aftermath of exposure. It is unlikely to be effective more than 5 days after exposure, as demonstrated in rabbit models.[45][46]
Signs and symptoms
All documented human infections with B virus to date have been symptomatic. However, there have been only about 50 documented cases of B virus infection published to date, 21 of which have been fatal.[4] Thus, limited numbers preclude solid data about typical presentation, particularly for early signs and symptoms.
Disease onset in humans typically occurs within 5 weeks of exposure, but may be early and abrupt (3-7 days).[3] Clinical presentation of a B virus-infected person may vary, and patients may progress rapidly to CNS symptoms. Patients with B virus infection should have a history of risk factors for infection and may show one or more of the following signs and/or symptoms at initial presentation, depending on the time post-exposure and the site and nature of infection:
Systematic signs/symptoms
Fever (≥37.5°C) and/or chills
Non-specific flu-like symptoms (e.g., malaise, fatigue, listlessness)
Lymphadenitis associated with site of infection
Lymphangitis associated with site of infection
Nausea/vomiting
Abdominal pain
Headache (may indicate CNS involvement)
Focal neurological signs/symptoms
Paraesthesia at the site of infection
Paraesthesia in the lower limbs
Hyperaesthesias
Ocular pain (for infections resulting from splash exposure of the eye or reactivation via the optic nerve)
Persistent hiccups (suggests neurological involvement at the level of the diaphragm)
Muscle weakness (paresis)
Central neurological signs/symptoms (CNS signs/symptoms are most alarming)
Severe persistent headache
Dizziness
Dyspnoea
Gait disturbances (if present presume CNS origin)
Ataxia (if present presume CNS origin)
Diplopia
Nystagmus
Agitation
Meningismus
Ascending or acute flaccid paralysis
Dermatological signs
Vesicular skin lesions at the site of infection (similar to those caused by herpes simplex virus infection)
Rash, possibly pruritic, at the site of exposure
Conjunctivitis.
Numbness and/or tingling at the site of exposure and/or flu-like symptoms (e.g., fever, muscle aches, fatigue, malaise, and listlessness with or without nausea and vomiting) tend to occur first. Some patients may also present initially with vesicular skin lesions at the site of infection, rash that may be pruritic, lymphadenitis (inflamed lymph nodes), lymphangitis (inflammation of lymphatic vessels), abdominal pain, or persistent hiccups (which may suggest neurological disease at the level of the diaphragm).
Neurological signs (e.g., meningismus, hyperaesthesias, ataxia, diplopia, agitation, and ascending or acute flaccid paralysis) may develop as the virus progresses through the peripheral nervous system to infect the CNS. CNS involvement may progress rapidly to paralysis and death, even with antiviral therapy and supportive care. Respiratory failure, often associated with ascending paralysis, is a common cause of death in those with CNS involvement. Those who survive usually have long-term neurological sequelae.
Specimen collection
Specimen collection for serological analysis should be done when there has been an exposure to macaque body fluid regardless of the risk assessment of the exposure, and regardless of whether or not the patient is receiving PEP.
In asymptomatic patients, acute serum should be collected immediately after an exposure (at initial evaluation) to establish a baseline profile, and again at 3 to 6 weeks after the exposure to evaluate whether or not seroconversion to B virus has occurred or, when the baseline is not negative, whether a substantial (≥4-fold) rise in antibody titre has occurred.[3][4] In symptomatic patients, it may be informative to collect serial specimens for serological testing.
Prophylactic antiviral drugs may suppress seroconversion and delay onset of symptoms without preventing infection. Therefore, if antiviral drugs are administered as prophylaxis, an acute serum sample should be taken at baseline, and convalescent samples should be taken at 3 to 6 weeks and 3 months post-exposure (and potentially serially, thereafter) because seroconversion may be delayed. The patient should be followed during this time for onset of symptoms compatible with B virus infection. Patients in whom prophylactic antivirals have been discontinued should be closely monitored for onset of signs and symptoms that suggest B virus infection.[4]
Collection of wound specimens at the time of exposure is not recommended. If specimens for PCR or viral culture are collected at the time of exposure, they should be taken from the site of exposure only after it has been thoroughly cleansed in order to reduce the risk of forcing the virus more deeply into the wound. Specimens for PCR or culture are usually only obtained if clinical symptoms are present, or if antibody-positive serology suggests infection. It should also be recognised that specimens collected for PCR or culture from the site of exposure at the time of exposure are often negative due to removal of free viral particles during cleansing and, therefore, may lack diagnostic or clinical significance for infection.
Specimens must be sent to specialist diagnostic laboratories with expertise in testing for B virus. B virus is a Risk Group 4 agent requiring a biosafety level class 4 (BSL-4) laboratory for handling; therefore, the specialist laboratory must have BSL-4 biocontainment facilities as well as established capacity and certification to perform diagnostics for B virus.
Initial laboratory investigations
Serological analysis performed in an expert BSL-4 laboratory is the standard first-line test for diagnosing B virus infections. It is also the most effective (and cost effective) laboratory evaluation for following exposed patients. When B virus infection is suggested by serology, infection is ideally confirmed using PCR of specimens collected from herpetic lesions, or, in the absence of infected sites, conjunctiva, buccal mucosa, or cerebrospinal fluid (CSF). Virus culture remains an option for confirming B virus infection, but its utility in guiding clinical management is limited. Furthermore, there are potential hazards associated with growing the virus in the laboratory; an expert laboratory with BSL-4 capabilities is required. A positive culture must be confirmed using an agent-specific test (e.g., PCR, sequencing, restriction endonuclease analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] analysis of viral proteins). PCR is preferred as it is faster, more sensitive, has a lower hazard risk, and is more cost effective than culture. Thus, serological analysis with confirmation by PCR is a more feasible clinical approach. If a patient is symptomatic, PCR should be the first investigation to order. Culture is not required for clinical confirmation, but it may be helpful in confirming the presence of viable virus and for assessment of drug sensitivity.[47]
Serological analysis:
Serological testing should be carried out to evaluate for B virus infection, but will not inform immediate treatment decisions owing to the time required to develop detectable virus-specific antibodies. Immediate treatment decisions must be made based on clinical judgement.
Testing of paired acute and convalescent serological specimens will be most meaningful, but testing of single specimens might be considered if clinical signs or symptoms of B virus infection are present.
Documentation of seroconversion or of a ≥4-fold rise in antibody titre strongly suggests that exposure has progressed to infection. Alternatively, the absence of either of these findings by serology at 3 to 6 weeks following exposure can provide some reassurance of the absence of infection.
Although seroconversion temporally associated with B virus exposure can identify infection with relative confidence, cross-reactions with antibodies from other herpes viruses, particularly HSV-1, can occur. This can complicate diagnostic interpretation through false-positive reactions.[48] Thus, serological rises in titre not definitively correlated with B virus exposure might identify a new infection, or seropositivity not directly associated with a new exposure may identify a past infection, but both are less definitive than seroconversion after exposure.
People with a history of possible exposure should not be diagnosed as infected on the basis of serological reactivity alone in the absence of symptomatic disease or laboratory confirmation of virus shedding (e.g., by PCR or culture). Expert assistance with diagnostic testing and interpretation is essential.
PCR and viral culture:
In the absence of established infection, a positive PCR or viral culture from samples collected from the exposed area shortly after exposure indicates that B virus exposure has occurred. However, a negative result does not rule out exposure, and neither positive nor negative results can identify whether an exposure has progressed to infection. Thus, results of these samples have limited diagnostic value.
In symptomatic patients, specimens collected for PCR or culture from skin lesions or elsewhere do have clinical value as they may establish the diagnosis, document infection, and inform both clinical management of the patient and infection control practices. For example, a specimen for PCR or culture collected from sites associated with the appearance of early symptoms such as vesicular skin lesions or distant sites implicated by clinical syndromes (e.g., the CSF when CNS infection is suspected) is more likely to produce a positive result and can confirm an initial diagnosis that was based on serological testing and clinical presentation.[4]
A positive PCR test or culture at the exposure site at a time distant from exposure or at a site not exposed to the virus (e.g., lesions, conjunctivae, oropharynx, CSF) at any time and in association with symptoms concordant with B virus disease indicates infection.[4]
In a setting with clinical symptoms compatible with infection and seropositivity supporting B virus infection, PCR is adequate to confirm the diagnosis of B virus infection.
Symptomatic patients who have been documented to be PCR or culture positive for B virus should have the tests repeated weekly until two consecutive negatives have been obtained to monitor treatment response and confirm viral shedding has ceased.
During the follow-up period, samples for PCR can be obtained from crusted lesions. Samples from lesions that have not yet crusted can be used for culture.
Other investigations to consider
If possible and if safe to do so, and under veterinary consultation, laboratory testing (e.g., serological analysis, PCR, culture) of the animal responsible for exposure can be considered to identify B virus infection. Decisions to test animals associated with possible B virus exposures should take into consideration that, while test results from the animal may inform management of that animal or the larger troop from which it comes, they are unlikely to substantially inform patient management. Decisions on the evaluation and management of patients must be initiated before these results will be available and continued based on the evidence of the patient, not the exposure source. Thus, clinical management of the patient will not justify the cost or risk of culture of animals associated with possible exposures.
Pregnancy testing should be carried out in women of childbearing age with suspected or known B virus exposure to aid in the decision of which antiviral drugs to choose. Experience with aciclovir in pregnancy is limited but there is no evidence of an increased risk to the developing fetus. Experience with other antivirals is less extensive.[4]
Brain MRI can be performed to look for evidence of encephalomyelitis if neurological symptoms develop.[27][28][49] A CT scan of the brain can be performed if MRI is unavailable, but CT is less sensitive than MRI for brainstem encephalitis; to have utility it should be done with contrast.
If neurological symptoms develop, a lumbar puncture should be performed and CSF analysed for routine chemistry and cell counts, and for identification of B virus (e.g., culture, PCR, or serology).[50][51]
Under neurological consultation, EEG can be considered if needed to further characterise the encephalitis. B virus encephalitis usually presents as a brainstem encephalitis that becomes diffuse over time, in contrast to HSV encephalitis which is usually a unilateral temporal lobe encephalitis. Both HSV and B virus encephalitis are treated with intravenous aciclovir so treatment should not be delayed. Treatment should be initiated at the higher B virus levels, and adjusted if subsequent evaluation suggests HSV is the aetiology, or consideration should be made to switch to ganciclovir if evaluation suggests a B virus aetiology.
Guided by neurological consultation, additional useful information about brainstem or upper spinal cord function may be obtained with brainstem auditory evoked responses (patient conscious) or somatosensory evoked potentials (patient unconscious).
Use of this content is subject to our disclaimer