Aetiology
EEEV is an arthropod-borne virus belonging to the family Togaviridae and to the genus Alphavirus (formerly Group A arboviruses), and is responsible for causing eastern equine encephalitis. EEEV is a lipid-membrane encapsulated positive-sense single-stranded RNA virus with icosahedral symmetry. The virus particles are spherical with a diameter of 60 to 65 nm.[15] Four genetic lineages of EEEV have been identified (classified I-IV). Group I occurs in North America and the Caribbean, and causes the majority of human disease, while groups II to IV (now known collectively as Madariaga virus [MADV]) are found in Central and South America, and mainly cause equine disease.[3] The North American and South American lineages are genetically divergent, in keeping with differences seen in virulence and transmission.[3] MADV was thought to be avirulent in humans; however, in 2010, the first outbreak of this virus in humans was reported in Darién, Panama.[16] Studies examining this outbreak are now ongoing.
North American EEEV is maintained in an enzootic transmission cycle involving avian hosts, mainly passerine birds, and the mosquito vector Culiseta melanura.[5] The virus incubates in the mosquito for 1 week after it has become infected by feeding on the blood of a viraemic host. Transmission of the virus occurs when the infected mosquito feeds on the blood of an uninfected host (e.g., another bird); the C melanura mosquito feeds almost exclusively on avian species. The key avian species involved in the natural transmission cycle of EEEV include the wood thrush and the American robin.[20] During epizootic cycles, ‘bridge’ vectors (e.g., mosquitoes from the genus Aedes, Coquillettidia, and Culex) spread the virus to other avian hosts and non-avian hosts such as horses and humans.
The transmission cycle of South American EEEV (MADV) is not fully understood, but it is possible that rodents serve as amplifying enzootic hosts, and Culex mosquito species of the subgenus Melanoconion act as vectors.[17]Culex (Melanoconion) pedroi is thought to be the main mosquito vector for EEEV in South America,[18] whereas Culex (Melanoconion) taeniopus is thought to be the main mosquito vector in Central America.[21]
As well as the natural transmission of EEEV via the mosquito vector, laboratory-acquired infections may occur via aerosol inhalation, accidental subcutaneous inoculation (e.g., bite from an infected animal), and contact of the virus with broken skin or mucosal membranes.[1] There is no evidence for human-to-human transmission. Furthermore, infected humans do not generally develop sufficient viraemia to infect mosquitoes, therefore do not contribute to the transmission cycle, thus making humans a ‘dead end’ host for EEEV.
[Figure caption and citation for the preceding image starts]: This diagram illustrates the methods by which the arbovirus EEEV reproduces and amplifies itself in avian populations within the US, and is subsequently transmitted to human beings and horses (the 'dead end' hosts) through the bite of a number of different mosquito speciesCDC Public Health Image Library (PHIL) [Citation ends].
Pathophysiology
EEEV is transmitted to humans and other hosts from the bite of an infectious mosquito (e.g., Culiseta, Culex, or Coquillettidia species). EEEV has a single-stranded, positive-sense RNA genome that encodes four nonstructural proteins (nsP1-4), and five structural proteins (E1, E2, E3, 6K/TF, and CP [capsid protein]) for replication and pathogenesis in its host. E2 is a key glycoprotein that is present in the lipid envelope of the virus and is responsible for binding to host cell receptors. Upon subcutaneous inoculation by an infected mosquito, the virus first binds and replicates in Langerhans cells and cells of mesenchymal origin in the skin. Dendritic cells in the skin also become infected. These infected cells transport the virus to the lymph nodes where it infects other immune cells, as well as the neurological cells of the central nervous system (CNS).[22][23] The virus also has tropism for mesenchymal cells, such as osteoblasts and fibroblasts.[24] The virus enters the host cell via clathrin-mediated endocytosis, and undergoes fusion of the viral envelope, disassembly of the core, and release of genomic RNA. Genomic RNA is used for translation of proteins and transcription of RNA. Viral proteins are transported to the cell surface, where conformational changes in the envelope proteins drive the budding process and release of virions.[25]
The incubation period for EEEV in humans ranges from 4 to 10 days.[15] In early infection, type I interferon plays a prominent role in the ability of the virus to replicate; the ability to respond to type I interferon is a determinant of disease severity.[26] It has also been observed that pathogenic alphaviruses, such as EEEV, use the secondary structural motif within the 5’ untranslated region of their RNA to impede the cellular mechanisms responsible for the antiviral effects of type I interferon.[27] Subsequent adaptive humoral responses include the production of IgM, followed by IgG. Neutralising and non-neutralising antibodies against epitopes on E1, E2, and E3 glycoproteins have been shown to protect against infection, and promote alphavirus recovery in murine studies.[23] Neutralising antibodies mainly target the E2 glycoprotein, which prevents the virus from binding to host cells. Recovery is also mediated by T cells, as demonstrated by the ability of mice lacking antibody to clear virus.[23]
Pathological findings of the CNS include cerebral oedema and acute and chronic perivascular inflammation within the cortex, thalamus, basal ganglia, and brainstem. Neuronal injury and death are prominent histological findings.[2][Figure caption and citation for the preceding image starts]: Transmission electron micrograph showing EEEV virions in a tissue specimenCDC Public Health Image Library (PHIL) [Citation ends].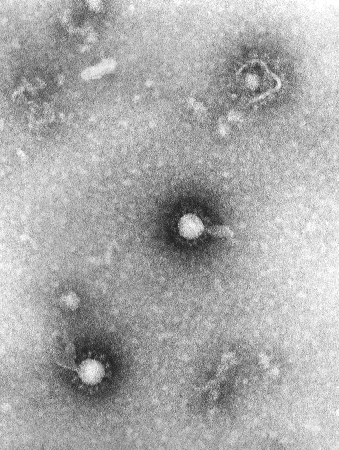
Classification
Baltimore Classification
Group IV: positive-sense single-stranded RNA viruses
Eastern equine encephalitis virus (EEEV) subtypes
There are four genetic lineages of EEEV:[3]
Lineage I
Endemic in North America and the Caribbean
Associated with severe clinical disease in humans.
Lineage II to IV (now collectively known as Madariaga virus)
Endemic in South and Central America
Mainly responsible for equine disease.
Use of this content is subject to our disclaimer