Anaphylaxis
- Overview
- Theory
- Diagnosis
- Management
- Follow up
- Resources
Treatment algorithm
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups: see disclaimer
Look out for this icon: for treatment options that are affected, or added, as a result of your patient's comorbidities.
presumed anaphylaxis: in cardiorespiratory arrest
CPR and advanced life support
If cardiorespiratory arrest occurs, start CPR according to your local advanced life support guideline and call for help.[74]Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council guidelines 2021: adult advanced life support. Resuscitation. 2021 Apr;161:115-51. https://www.doi.org/10.1016/j.resuscitation.2021.02.010 http://www.ncbi.nlm.nih.gov/pubmed/33773825?tool=bestpractice.com [92]Resuscitation Council (UK). Adult advanced life support. May 2021 [internet publication]. https://www.resus.org.uk/library/2021-resuscitation-guidelines/adult-advanced-life-support-guidelines [93]Resuscitation Council (UK). Paediatric advanced life support. Mar 2024 [internet publication]. https://www.resus.org.uk/library/2021-resuscitation-guidelines/paediatric-advanced-life-support-guidelines [94]Van de Voorde P, Turner NM, Djakow J, et al. European Resuscitation Council guidelines 2021: paediatric life support. Resuscitation. 2021 Apr;161:327-87. https://www.doi.org/10.1016/j.resuscitation.2021.02.015 http://www.ncbi.nlm.nih.gov/pubmed/33773830?tool=bestpractice.com
Do not give intramuscular adrenaline (epinephrine) after cardiac arrest has occurred as circulation to the muscle, and therefore distribution, is inadequate.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Some patients who suffer a cardiorespiratory arrest following anaphylaxis and who regain spontaneous circulation may need ongoing critical care input.
Following discussion with a senior clinician and the critical care team, you may be able to continue the management of other patients in the resuscitation area of the emergency department as per below.
presumed anaphylaxis: not in cardiorespiratory arrest
1st line – concurrently assess and treat using ABCDE principles
concurrently assess and treat using ABCDE principles
Anaphylaxis is a medical emergency that has varied presentations. Treat the greatest threat to life first, but be aware that positioning the patient, removing the trigger, and giving adrenaline (epinephrine) take seconds, while establishing an airway may take longer and require specialist expertise.
The order of steps presented here is designed for a single responder; however, several rescuers should undertake multiple actions simultaneously.
Call for help immediately so that you can give intramuscular adrenaline at the same time as positioning the patient and removing the trigger.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
The Resuscitation Council (UK) recommends calling for help immediately, whereas the European Academy for Allergy and Clinical Immunology recommends giving adrenaline before calling for help.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication].
https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
[59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77.
https://onlinelibrary.wiley.com/doi/10.1111/all.15032
http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
[Figure caption and citation for the preceding image starts]: Anaphylaxis diagnostic and management flowchart. See Management section for further information, including actions to take if there is no improvement in breathing or circulation problems after two doses of adrenaline.Created by the BMJ Knowledge Centre; adapted with permission from the Resuscitation Council (UK). Emergency treatment of anaphylaxis: guidelines for healthcare providers. May 2021 [Citation ends].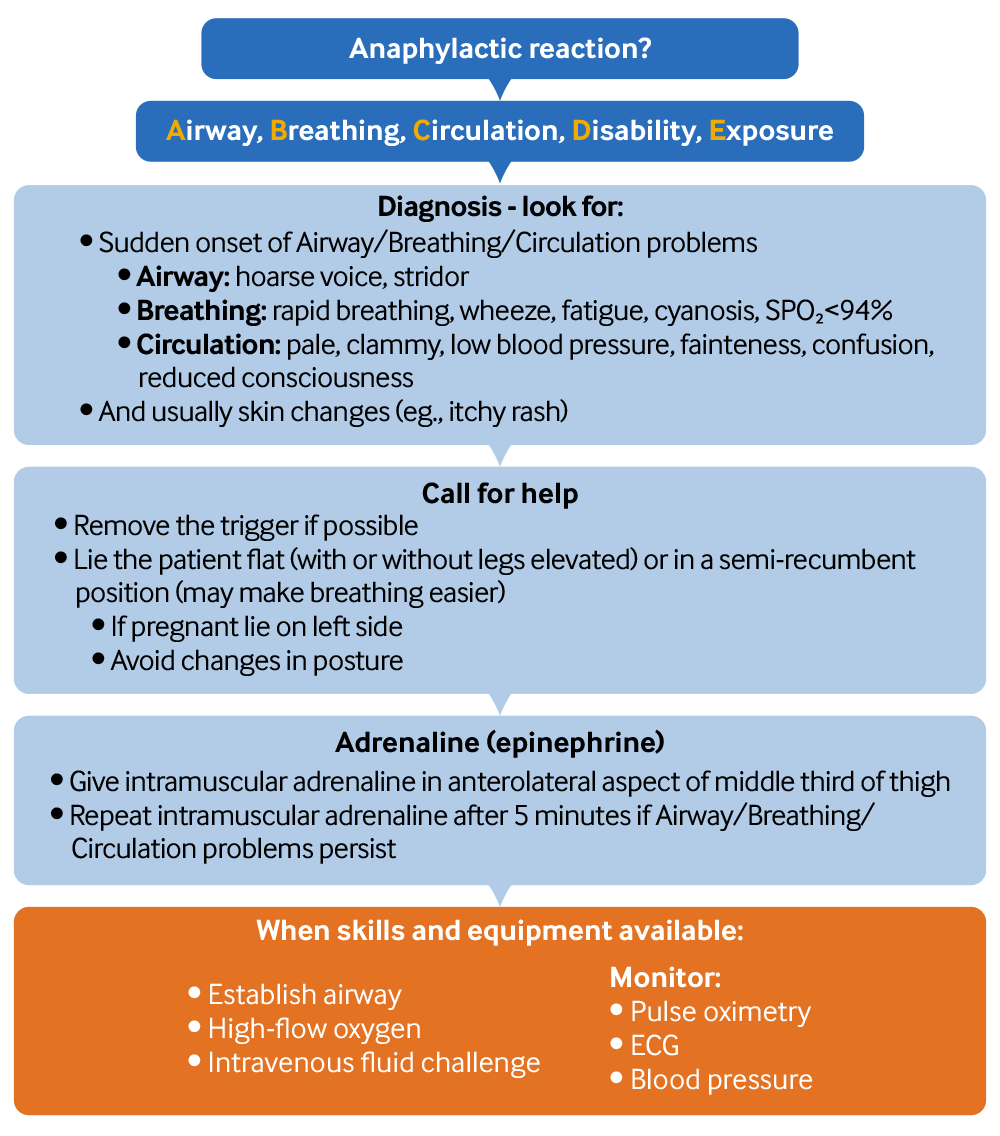
position the patient and remove the trigger
Treatment recommended for ALL patients in selected patient group
Position the patient comfortably. Keep the patient in a supine or semi-recumbent position during treatment.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment If the patient: [32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
Feels faint: do not sit or stand them up as this can cause cardiac arrest
Has predominant airway/breathing problems: place them in a semi-recumbent position, or sitting up with elevated legs, to make breathing easier
Has predominant circulation problems: lie them flat with/without legs up
Is unconscious and breathing: position on their side (recovery position)
Is pregnant: lie them on their left side or with the bed in a head-down position. In practice, this is only necessary in an obviously gravid uterus well into the pregnancy.
Avoid sudden changes in posture.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Attempt to remove the trigger, but do not delay treatment if this is not possible.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Stop any drug that may be the cause of the anaphylaxis (e.g., drug infusion, blood products).
Remove the stinger after a bee sting. Early removal is more important than the method of removal.
Do not try to make the patient vomit.
adrenaline (epinephrine)
Treatment recommended for ALL patients in selected patient group
Give intramuscular adrenaline into the anterolateral aspect of the middle third of the thigh, using a needle long enough to reach the muscle.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [122]Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001 Nov;108(5):871-3. http://www.ncbi.nlm.nih.gov/pubmed/11692118?tool=bestpractice.com
Length of needle[97]Song TT, Nelson MR, Chang JH, et al. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol. 2005 May;94(5):539-42. http://www.ncbi.nlm.nih.gov/pubmed/15945556?tool=bestpractice.com
25 mm (blue 23G or orange 25G) needle is best and suitable for all ages
16 mm (orange 25G) needle is appropriate for preterm or small infants
38 mm (green 21G) needle may be needed in some adults.
Injection technique[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Stretch the skin and give the injection with the needle at a 90° angle to the skin.
While there are no absolute contraindications to giving adrenaline for anaphylaxis, both underlying comorbidity and concomitant medications may complicate the treatment of anaphylaxis in older people and people with cardiovascular disease. Seek advice as early as possible in these patients, but do not delay giving adrenaline in order to do so.[63]McLean-Tooke AP, Bethune CA, Fay AC, et al. Adrenaline in the treatment of anaphylaxis: what is the evidence? BMJ. 2003 Dec 6;327(7427):1332-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC286326 http://www.ncbi.nlm.nih.gov/pubmed/14656845?tool=bestpractice.com
Practical tip
In hospital practice, if you give adrenaline, call for senior assistance in case the patient deteriorates.
Seek advice from a cardiologist as soon as you become aware that a patient has cardiovascular disease (CVD) but do not delay giving adrenaline in a life-threatening anaphylaxis when adrenaline is required.
Treatment is complex because:
CVD limits cardiac reserve, which might compound hypotension
Patients with CVD may be on beta-blockers, which may counteract the effects of adrenaline by limiting heart rate, compromising cardiac output, and opposing its bronchodilatory properties. This may result in failure to reverse anaphylaxis
Hypotension, tachycardia, and adrenaline may cause myocardial ischaemia by reducing perfusion during diastole
The alpha-1 agonist action of adrenaline can lead to severe hypertension/hypertensive crisis.
Repeat the intramuscular dose of adrenaline if there is no improvement after 5 minutes.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
If the patient’s respiratory or cardiovascular symptoms do not improve after two doses of intramuscular adrenaline (refractory anaphylaxis) seek urgent expert help to allow for a low-dose, intravenous adrenaline infusion to be started by a specialist.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment See Intravenous fluids below for more detail.
Intravenous adrenaline boluses are not recommended unless the patient is in cardiac arrest (but they may be used by specialists experienced in the use and titration of vasopressors in their normal clinical practice, while the adrenaline infusion is set up).[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Continue to repeat intramuscular adrenaline after every 5 minutes until the infusion has been started.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Intravenous adrenaline should only be administered by a specialist with experience in the use and titration of vasopressors in their normal clinical practice (e.g., anaesthetists, emergency physicians, intensive care doctors, or, in the case of children, paediatric anaesthetists, paediatric emergency physicians, paediatric intensivists).[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Many healthcare providers have given intravenous adrenaline as part of cardiac arrest resuscitation, but this is insufficient experience to safely use intravenous adrenaline to treat anaphylaxis.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Be aware that patients on beta-blockers may be less responsive to adrenaline.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
The low-dose intravenous adrenaline infusion should be continued and titrated as symptoms improve. Monitor for adrenaline side effects: tachycardia, arrhythmia, hypertension. If present, the infusion rate should be reduced (or stopped if side effects are severe).[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Practical tip
Be aware that patients with bradykinin-mediated angio-oedema may appear to have anaphylaxis, but they will not respond to adrenaline. Instead, they need C1-esterase inhibitor concentrate, icatibant (a bradykinin B2 receptor antagonist), or fresh frozen plasma. Seek expert help urgently.
Primary options
adrenaline (epinephrine): children <6 months of age: 100-150 micrograms (0.1 to 0.15 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children 6 months to 6 years of age: 150 micrograms (0.15 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children 6-12 years of age: 300 micrograms (0.3 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children >12 years of age and adults: 500 micrograms (0.5 mL) intramuscularly initially, may repeat at 5-minute intervals according to response
More adrenaline (epinephrine)Intravenous adrenaline may be an alternative option in some circumstances. It should only be administered by a specialist with experience in the use and titration of vasopressors. Consult a specialist for guidance on intravenous dose.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
These drug options and doses relate to a patient with no comorbidities.
Primary options
adrenaline (epinephrine): children <6 months of age: 100-150 micrograms (0.1 to 0.15 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children 6 months to 6 years of age: 150 micrograms (0.15 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children 6-12 years of age: 300 micrograms (0.3 mL) intramuscularly initially, may repeat at 5-minute intervals according to response; children >12 years of age and adults: 500 micrograms (0.5 mL) intramuscularly initially, may repeat at 5-minute intervals according to response
More adrenaline (epinephrine)Intravenous adrenaline may be an alternative option in some circumstances. It should only be administered by a specialist with experience in the use and titration of vasopressors. Consult a specialist for guidance on intravenous dose.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
adrenaline (epinephrine)
establish airway plus high-flow oxygen
Treatment recommended for ALL patients in selected patient group
Follow basic or advanced life support principles.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Practical tip
Be aware of the potential for impending airway difficulties. If there is airway obstruction at presentation, basic manoeuvres to maintain the airway may be required immediately. Seek help from an anaesthetist early if you are worried about maintaining the airway.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Give high-concentration oxygen at a high flow of >10 L/minute with a mask and oxygen reservoir.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
The Resuscitation Council (UK) and the British Thoracic Society recommend a target oxygen saturation of >94% to 98%.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
However, evidence suggests that liberal use of supplemental oxygen (target SpO 2 >96%) in acutely ill adults is associated with higher mortality than more conservative oxygen therapy.[99]Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018 Apr 28;391(10131):1693-705. http://www.ncbi.nlm.nih.gov/pubmed/29726345?tool=bestpractice.com
A lower target SpO 2 of 88% to 92% is appropriate if the patient is at risk of hypercapnic respiratory failure.[98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
intravenous fluids
Treatment recommended for ALL patients in selected patient group
Establish intravenous access as soon as possible.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
If you cannot obtain intravenous access, attempt intraosseous access if you are trained to do so.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Give a rapid intravenous fluid bolus (over less than 15 minutes) with a non-glucose-containing crystalloid that has a sodium concentration in the range of 130 to 154 mmol/L (130 to 154 mEq/L), early in the presence of hypotension, shock, or if there is a poor response to an initial dose of intramuscular adrenaline.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [100]National Institute for Health and Care Excellence. Intravenous fluid therapy in adults in hospital. May 2017 [internet publication]. https://www.nice.org.uk/guidance/cg174
Adults: 500-1000 mL
Children: 10 mL/kg.
Check local protocols for specific recommendations on fluid choice. There is debate, based on conflicting evidence, on whether there is a benefit in using normal saline or balanced crystalloid in critically ill patients.
Practical tip
Be aware that large volumes of normal saline as the sole fluid for resuscitation may lead to hyperchloraemic acidosis.
Also note that use of lactate-containing fluid in a patient with impaired liver metabolism may lead to a spuriously elevated lactate level, so results need to be interpreted with other markers of volume status.
Evidence: Choice of fluids
Evidence from two large randomised controlled trials (RCTs) suggests there is no difference between normal saline and a balanced crystalloid for critically ill patients in mortality at 90 days, although results from two meta-analyses including these RCTs point to a possible small benefit of balanced solutions compared with normal saline.
There has been extensive debate over the choice between normal saline (an unbalanced crystalloid) versus a balanced crystalloid (such as Hartmann’s solution [also known as Ringer’s lactate] or Plasma-Lyte®). Clinical practice varies widely, so you should check local protocols.
In 2021 to 2022 two large double blind RCTs were published assessing intravenous fluid resuscitation in intensive care unit [ICU] patients with a balanced crystalloid solution (Plasma-Lyte) versus normal saline. The Plasma-Lyte 148 versus Saline (PLUS) trial (53 ICUs in Australia and New Zealand; N= 5037) and the Balanced Solutions in Intensive Care Study (BaSICS) trial (75 ICUs in Brazil; N=11,052).[126]Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021 Aug 10;326(9):1-12. http://www.ncbi.nlm.nih.gov/pubmed/34375394?tool=bestpractice.com [127]Finfer S, Micallef S, Hammond N, et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N Engl J Med. 2022 Mar 3;386(9):815-26. http://www.ncbi.nlm.nih.gov/pubmed/35041780?tool=bestpractice.com
In the PLUS study 45.2% of patients were admitted to ICU directly from surgery (emergency or elective), 42.3% had sepsis and 79.0% were receiving mechanical ventilation at the time of randomisation.
In BaSICS almost half the patients (48.4%) were admitted to ICU after elective surgery and around 68.0% had some form of fluid resuscitation before being randomised.
Both found no difference in 90-day mortality overall or in pre-specified subgroups for patients with acute kidney injury (AKI), sepsis or post-surgery. They also found no difference in the risk of AKI.
In BaSICS, for patients withtraumatic brain injury, there was a small decrease in 90-day mortality with normal saline - however, the overall number of patients was small (<5% of total included in the study) so there is some uncertainty about this result. Patients with traumatic brain injury were excluded from PLUS as the authors felt these patients should be receiving saline or a solution of similar tonicity.
One meta-analysis of 13 RCTs (including PLUS and BaSICS) confirmed no overall difference, although the authors did highlight a non-significant trend towards a benefit of balanced solutions for risk of death.[128]Hammond DA, Lam SW, Rech MA, et al. Balanced crystalloids versus saline in critically ill adults: a systematic review and meta-analysis. Ann Pharmacother. 2020 Jan;54(1):5-13. https://journals.sagepub.com/doi/full/10.1177/1060028019866420 http://www.ncbi.nlm.nih.gov/pubmed/31364382?tool=bestpractice.com
One subsequent individual patient data meta-analysis included six RCTs of which only PLUS and BaSICS were assessed as being at low risk of bias. There was no statistically significant difference in in-hospital mortality (OR 0·96, 95% CI 0·91 to 1·02). However, the authors argued that using a Bayesian analysis there was a high probability that balanced solutions reduced in-hospital mortality, although they acknowledged that the absolute risk reduction was small.[129]Zampieri FG, Cavalcanti AB, Di Tanna GL, et al. Balanced crystalloids versus saline for critically ill patients (BEST-Living): a systematic review and individual patient data meta-analysis. Lancet Respir Med. 2024 Mar;12(3):237-46. http://www.ncbi.nlm.nih.gov/pubmed/38043564?tool=bestpractice.com
A pre-specified subgroup analysis of patients with traumatic brain injury (N=1961) found that balanced solutions increased the risk of in-hospital mortality compared with normal saline (OR 1·42, 95% CI 1·10 to 1·82).
Previous evidence has been mixed.
One 2015 double-blind, cluster randomised, double-crossover trial conducted in four ICUs in New Zealand (N=2278), the 0.9% Saline vs Plasma-Lyte for ICU fluid Therapy (SPLIT) trial, found no difference for in-hospital mortality, AKI, or use of renal-replacement therapy.[130]Young P, Bailey M, Beasley R, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015 Oct 27;314(16):1701-10. https://jamanetwork.com/journals/jama/fullarticle/2454911 http://www.ncbi.nlm.nih.gov/pubmed/26444692?tool=bestpractice.com
However, one 2018 US multicentre unblinded cluster-randomised trial - the isotonic Solutions and Major Adverse Renal events Trial (SMART), among 15,802 critically ill adults receiving ICU care - found possible small benefits from balanced crystalloid (Ringer’s lactate or Plasma-Lyte) compared with normal saline. The 30-day outcomes showed a non-significant reduced mortality in the balanced crystalloid group versus the normal saline group (10.3% vs 11.1%; OR 0.90, 95.% CI 0.80 to 1.01) and a major adverse kidney event rate of 14.3% versus 15.4% respectively (OR 0.91, 95% CI 0.84 to 0.99).[42]Lee JM, Greenes DS. Biphasic anaphylactic reactions in pediatrics. Pediatrics. 2000 Oct;106(4):762-6. http://www.ncbi.nlm.nih.gov/pubmed/11015520?tool=bestpractice.com
One 2019 Cochrane review included 21 RCTs (N=20,213) assessing balanced crystalloids versus normal saline for resuscitation or maintenance in a critical care setting.[131]Antequera Martín AM, Barea Mendoza JA, Muriel A, et al. Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev. 2019 Jul 19;(7):CD012247. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012247.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/31334842?tool=bestpractice.com
The three largest RCTs in the Cochrane review (including SMART and SPLIT) all examined fluid resuscitation in adults and made up 94.2% of participants (N=19,054).
There was no difference in in‐hospital mortality (OR 0.91, 95% CI 0.83 to 1.01; high quality evidence as assessed by GRADE), acute renal injury (OR 0.92, 95% CI 0.84 to 1.00; GRADE low), or organ system dysfunction (OR 0.80, 95% CI 0.40 to 1.61; GRADE very low).
Repeat fluid boluses if necessary.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment Large amounts of fluid may be needed.
Use a non-glucose-containing crystalloid in preference to 0.9% sodium chloride to reduce the risk of hyperchloraemic acidosis.
Decrease or stop the fluid infusion if signs of fluid overload develop.
Practical tip
Differentiating fluid overload from signs and symptoms of anaphylaxis:
Shortness of breath, peripheral oedema, raised jugulovenous pressure, a third heart sound, and inspiratory crackles in the lungs on auscultation indicate cardiac failure, but many of these symptoms and signs overlap with those of anaphylaxis
Newly raised jugulovenous pressure, new respiratory crackles, peripheral oedema, and chest x-ray may help identify fluid overload more specifically.
Seek expert help to improve tissue perfusion with inotropes or vasopressors if needed.
Give an initial rapid fluid bolus and maintenance fluid therapy with adrenaline therapy to patients with refractory anaphylaxis (i.e, no improvement in respiratory or cardiovascular symptoms despite two doses of intramuscular adrenaline).[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
serial cardiorespiratory assessments
Treatment recommended for ALL patients in selected patient group
Monitor vital signs early.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
In all critically ill patients, as soon as possible, attach a:
Pulse oximeter
ECG monitor
Non-invasive blood pressure monitor.
nebulised adrenaline (epinephrine)
Additional treatment recommended for SOME patients in selected patient group
If there is marked stridor, give nebulised adrenaline in addition to intramuscular adrenaline.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [56]Muraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy. 2007 Aug;62(8):857-71. http://www.ncbi.nlm.nih.gov/pubmed/17590200?tool=bestpractice.com [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
Ensure oxygen levels are maintained if a patient needs a nebulised bronchodilator such as adrenaline.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Nebulisers should be driven by piped oxygen or from an oxygen cylinder fitted with a high-flow regulator capable of delivering a flow rate of >6 L/minute.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
If the cylinder does not produce a flow of >6 L/minute, use an air-driven nebuliser (with electrical compressor) and give supplemental oxygen by nasal cannulae at 2 to 6 L/minute to maintain an appropriate oxygen saturation level.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Change the patient back to their usual oxygen mask or cannulae when nebuliser therapy is complete.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
In some countries a specific nebulised adrenaline formulation is available. However, this is not available in the UK, and the solution in the parenteral ampoules is used to give the nebulised dose. Consult local protocols.
Primary options
adrenaline (epinephrine): children and adults: consult specialist for guidance on nebulised dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
adrenaline (epinephrine): children and adults: consult specialist for guidance on nebulised dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
adrenaline (epinephrine)
nebulised short-acting beta-2 agonist
Additional treatment recommended for SOME patients in selected patient group
Give a nebulised short-acting beta-2 agonist such as salbutamol to relieve symptoms of bronchoconstriction or if there is wheezing on auscultation.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [54]Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000 Aug;30(8):1144-50. http://www.ncbi.nlm.nih.gov/pubmed/10931122?tool=bestpractice.com [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
Ensure oxygen levels are maintained if a patient needs a nebulised bronchodilator such as salbutamol.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Nebulisers should be driven by piped oxygen or from an oxygen cylinder fitted with a high-flow regulator capable of delivering a flow rate of >6 L/minute.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
If the cylinder does not produce a flow of >6 L/minute, use an air-driven nebuliser (with electrical compressor) and give supplemental oxygen by nasal cannulae at 2 to 6 L/minute to maintain an appropriate oxygen saturation level.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Change the patient back to their usual oxygen mask or cannulae when nebuliser therapy is complete.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [98]O'Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017 Jun;72(suppl 1):ii1-90. http://www.ncbi.nlm.nih.gov/pubmed/28507176?tool=bestpractice.com
Practical tip
Patients with asthma who have anaphylaxis
If the patient has signs of anaphylaxis in addition to wheeze, coughing, and shortness of breath, give adrenaline before administering an asthma reliever.[104]Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014 Jun 9;69(8):1008-25. https://www.doi.org/10.1111/all.12429 http://www.ncbi.nlm.nih.gov/pubmed/24909706?tool=bestpractice.com [125]Australasian Society of Clinical Immunology and Allergy. Acute management of anaphylaxis. 2023 [internet publication]. https://www.allergy.org.au/hp/papers/acute-management-of-anaphylaxis-guidelines Such additional signs include:[51]Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct;13(10):100472. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7607509 http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com
Hypotension
Itching
Urticaria
Angio-oedema
Abdominal pain.
This is because the asthma reliever will only address respiratory symptoms; adrenaline (epinephrine) addresses many of the pathological mechanisms occurring during anaphylaxis.
If the patient has persistent signs of bronchoconstriction (whether or not the patient has pre-existing asthma), see our Acute exacerbation of asthma topics for information on further bronchodilator therapy.
Primary options
salbutamol inhaled: children 1 month to 4 years of age: 2.5 mg inhaled via nebuliser every 20-30 minutes or when required; children 5-11 years of age: 2.5 to 5 mg inhaled via nebuliser every 20-30 minutes or when required; children ≥12 years of age and adults: 5 mg inhaled via nebuliser every 20-30 minutes or when required
These drug options and doses relate to a patient with no comorbidities.
Primary options
salbutamol inhaled: children 1 month to 4 years of age: 2.5 mg inhaled via nebuliser every 20-30 minutes or when required; children 5-11 years of age: 2.5 to 5 mg inhaled via nebuliser every 20-30 minutes or when required; children ≥12 years of age and adults: 5 mg inhaled via nebuliser every 20-30 minutes or when required
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
salbutamol inhaled
intravenous atropine
Additional treatment recommended for SOME patients in selected patient group
Consider giving intravenous atropine if the patient becomes bradycardic. Consult a cardiologist or senior clinician with experience in administering this drug.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [74]Soar J, Böttiger BW, Carli P, et al. European Resuscitation Council guidelines 2021: adult advanced life support. Resuscitation. 2021 Apr;161:115-51. https://www.doi.org/10.1016/j.resuscitation.2021.02.010 http://www.ncbi.nlm.nih.gov/pubmed/33773825?tool=bestpractice.com [109]Brown SG, Blackman KE, Stenlake V, et al. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J. 2004 Mar;21(2):149-54. https://emj.bmj.com/content/21/2/149.long http://www.ncbi.nlm.nih.gov/pubmed/14988337?tool=bestpractice.com
Primary options
atropine: children and adults: consult specialist for guidance on dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
atropine: children and adults: consult specialist for guidance on dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
atropine
intravenous glucagon
Additional treatment recommended for SOME patients in selected patient group
Consider giving an intravenous glucagon infusion if the patient is on a beta-blocker and is not responding to adrenaline (epinephrine) infusion and adequate fluid resuscitation.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [102]Thomas M, Crawford I. Best evidence topic report: glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J. 2005 Apr;22(4):272-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1726748/pdf/v022p00272a.pdf http://www.ncbi.nlm.nih.gov/pubmed/15788828?tool=bestpractice.com Consult a cardiologist or senior clinician with experience in administering this drug.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Primary options
glucagon: children and adults: consult specialist for guidance on dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
glucagon: children and adults: consult specialist for guidance on dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
glucagon
after initial treatment: not in cardiorespiratory arrest
antihistamine
Give a non-sedating oral antihistamine (e.g., cetirizine) after initial resuscitation, in preference to chlorphenamine which causes sedation, especially in patients with persisting skin symptoms (urticaria and/or angio-oedema).[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Evidence: Antihistamines
Guidelines recommend antihistamines as adjuvant treatment only to relieve itching, flushing, urticaria, angio-oedema, and nasal and eye symptoms after initial resuscitation.
Antihistamines (H1 antagonists) do not prevent or relieve upper airway obstruction, hypotension, or shock; however, histamine is released from mast cells and basophils in acute allergic inflammation, so it is logical to give antihistamines for such conditions.[132]Runge JW, Martinez JC, Caravati EM, et al. Histamine antagonists in the treatment of acute allergic reactions. Ann Emerg Med. 1992 Mar;21(3):237-42. http://www.ncbi.nlm.nih.gov/pubmed/1536481?tool=bestpractice.com [133]Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000 Nov;36(5):462-8. http://www.ncbi.nlm.nih.gov/pubmed/11054200?tool=bestpractice.com [134]Nurmatov UB, Rhatigan E, Simons FE, et al. H2-antihistamines for the treatment of anaphylaxis with and without shock: a systematic review. Ann Allergy Asthma Immunol. 2014 Feb;112(2):126-31. http://www.ncbi.nlm.nih.gov/pubmed/24468252?tool=bestpractice.com
A Cochrane systematic review (literature search last rerun in 2010) found no randomised controlled trials examining the benefit of antihistamines in people with anaphylaxis.[135]Sheikh A, Ten Broek VM, Brown-Simon GA, et al. H1-antihistamines for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev. 2007 Jan 24;(1):CD006160. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD006160.pub2/full http://www.ncbi.nlm.nih.gov/pubmed/17253584?tool=bestpractice.com
Nevertheless, despite an absence of evidence, the Resuscitation Council (UK), the World Allergy Organization, and the European Academy of Allergy and Clinical Immunology (EAACI) are consistent in recommending antihistamines to relieve itching, flushing, urticaria, angio-oedema, and nasal and eye symptoms associated with anaphylaxis.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [51]Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020 Oct;13(10):100472. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7607509 http://www.ncbi.nlm.nih.gov/pubmed/33204386?tool=bestpractice.com [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
There is little evidence to support the initial use of H2 antagonists (e.g., ranitidine, cimetidine) in anaphylaxis and they are not recommended.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [133]Lin RY, Curry A, Pesola GR, et al. Improved outcomes in patients with acute allergic syndromes who are treated with combined H1 and H2 antagonists. Ann Emerg Med. 2000 Nov;36(5):462-8. http://www.ncbi.nlm.nih.gov/pubmed/11054200?tool=bestpractice.com
Primary options
cetirizine: children <2 years of age: 250 micrograms/kg orally as a single dose; children 2-6 years of age: 2.5 to 5 mg orally as a single dose; children 6-11 years of age: 5-10 mg orally as a single dose; children ≥12 years of age and adults: 10-20 mg orally as a single dose
Secondary options
chlorphenamine: children <6 months of age: 250 micrograms/kg intramuscularly/intravenously as a single dose, maximum 2.5 mg/dose; children 6 months to 6 years of age: 2.5 mg intramuscularly/intravenously as a single dose; children >6-12 years of age: 5 mg intramuscularly/intravenously as a single dose; children >12 years of age and adults: 10 mg intramuscularly/intravenously as a single dose
More chlorphenamineDose may be repeated if necessary up to a maximum of 4 doses per day.
These drug options and doses relate to a patient with no comorbidities.
Primary options
cetirizine: children <2 years of age: 250 micrograms/kg orally as a single dose; children 2-6 years of age: 2.5 to 5 mg orally as a single dose; children 6-11 years of age: 5-10 mg orally as a single dose; children ≥12 years of age and adults: 10-20 mg orally as a single dose
Secondary options
chlorphenamine: children <6 months of age: 250 micrograms/kg intramuscularly/intravenously as a single dose, maximum 2.5 mg/dose; children 6 months to 6 years of age: 2.5 mg intramuscularly/intravenously as a single dose; children >6-12 years of age: 5 mg intramuscularly/intravenously as a single dose; children >12 years of age and adults: 10 mg intramuscularly/intravenously as a single dose
More chlorphenamineDose may be repeated if necessary up to a maximum of 4 doses per day.
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
cetirizine
Secondary options
chlorphenamine
Consider – corticosteroid (for refractory anaphylaxis or ongoing asthma/shock)
corticosteroid (for refractory anaphylaxis or ongoing asthma/shock)
Additional treatment recommended for SOME patients in selected patient group
Do not use corticosteroids routinely to treat anaphylaxis.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Consider giving corticosteroids after initial resuscitation for refractory reactions or ongoing asthma or shock. Do not give corticosteroids preferentially to adrenaline.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
In asthma, early corticosteroid treatment may be indicated where an acute asthma exacerbation may have contributed to the severity of anaphylaxis. Give corticosteroids by the oral route where possible.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Primary options
hydrocortisone sodium succinate: children: 4 mg/kg intravenously as an initial dose, maximum 200 mg/dose; adults: 200 mg intravenously as an initial dose
These drug options and doses relate to a patient with no comorbidities.
Primary options
hydrocortisone sodium succinate: children: 4 mg/kg intravenously as an initial dose, maximum 200 mg/dose; adults: 200 mg intravenously as an initial dose
Drug choice, dose and interactions may be affected by the patient's comorbidities. Check your local drug formulary.
Show drug information for a patient with no comorbidities
Primary options
hydrocortisone sodium succinate
review by a senior clinician and observation
Treatment recommended for ALL patients in selected patient group
If a patient has received adrenaline (epinephrine), a senior clinician should decide on further treatment and how long observation is required.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Beware of the potential for a biphasic reaction in patients who have had an anaphylactic reaction, and occurs in around 5% of patients.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment This usually occurs within 12 hours after the initial reaction.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment Manage patients in the same way as you would for an initial anaphylactic reaction.[52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134
Risk factors for biphasic reactions following anaphylaxis include:[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
More severe initial presentation of anaphylaxis
Initial reaction requiring more than one dose of adrenaline
Delay in adrenaline administration (>30-60 min from symptom onset)
History of a previous biphasic reaction.
Observation should occur in a clinical area with facilities for treating life-threatening airway, breathing, and circulation problems.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Follow your local protocol for observing patients following a suspected anaphylactic reaction.
Based on the available evidence, the Resuscitation Council (UK) recommends observation to monitor for biphasic reactions as follows:[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
For at least 2 hours after resolution of symptoms (consider fast-track discharge) if there is:
A good response (within 5-10 minutes) to a single dose of adrenaline given within 30 minutes of onset of reaction
and
Complete resolution of symptoms
and
The patient has unused adrenaline autoinjectors and has been trained how to use them
and
There is adequate supervision following discharge
For at least 6 hours after resolution of symptoms if 2 doses of intramuscular adrenaline were given or there was a previous biphasic reaction.
Some patients may be discharged after 2 hours despite needing 2 doses of intramuscular adrenaline, for example, following a supervised allergy challenge in a specialist setting.
For at least 12 hours following resolution of symptoms for patients with:
Severe reaction requiring >2 doses of adrenaline
Severe asthma or reaction involved severe respiratory compromise
The possibility of continuing absorption of the allergen (e.g., slow-release medicines)
Presentation late at night
Possible inability to respond to deterioration
Difficulty accessing emergency care.
The National Institute for Health and Care Excellence in the UK recommends observing patients after suspected anaphylaxis for 6 to 12 hours from the onset of symptoms, depending on their response to emergency treatment.[52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134 It also recommends that you:[52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134
Consider a shorter observation period if the reaction was controlled promptly and easily
Admit children younger than 16 years with suspected anaphylaxis to the paediatric service in hospital.
However, evidence suggests that this approach may miss over 50% of biphasic reactions.[139]Kraft M, Scherer Hofmeier K, Ruëff F, et al. Risk factors and characteristics of biphasic anaphylaxis. J Allergy Clin Immunol Pract. 2020 Nov-Dec;8(10):3388-95.e6. http://www.ncbi.nlm.nih.gov/pubmed/32763470?tool=bestpractice.com [140]Pumphrey RS, Roberts IS. Postmortem findings after fatal anaphylactic reactions. J Clin Pathol. 2000 Apr;53(4):273-6. https://www.doi.org/10.1136/jcp.53.4.273 http://www.ncbi.nlm.nih.gov/pubmed/10823122?tool=bestpractice.com [141]Lee S, Bellolio MF, Hess EP, et al. Time of onset and predictors of biphasic anaphylactic reactions: a systematic review and meta-analysis. J Allergy Clin Immunol Pract. 2015 May-Jun;3(3):408-16.e1-2. http://www.ncbi.nlm.nih.gov/pubmed/25680923?tool=bestpractice.com [142]Kim TH, Yoon SH, Hong H, et al. Duration of observation for detecting a biphasic reaction in anaphylaxis: a meta-analysis. Int Arch Allergy Immunol. 2019;179(1):31-6. http://www.ncbi.nlm.nih.gov/pubmed/30763927?tool=bestpractice.com
Before discharge from hospital, give clear instructions to patients to return to hospital if symptoms recur.
Advise patients to eat some food at least 1 hour prior to discharge to reduce the risk of subsequent symptoms after leaving hospital.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
Postural hypotension has been reported after both anaphylaxis and milder allergic reactions. Ask patients to stand up (or to sit upright, if possible, if they have an existing disability) and assess them for dizziness. Measure blood pressure if appropriate.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment
post-anaphylaxis
adrenaline (epinephrine) auto-injector
Follow your local protocol for prescribing adrenaline auto-injectors to patients who have had a suspected anaphylactic reaction.
The UK National Institute for Health and Care Excellence (NICE) recommends providing an adrenaline auto-injector as an interim measure to cover the period before the patient’s allergy clinic appointment.[52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134
Latest European and UK guidance recommends providing a prescription for a further two adrenaline auto-injectors, which the patient should carry at all times.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134 [143]European Medicines Agency. Adrenaline auto-injectors. August 2015 [internet publication]. https://www.ema.europa.eu/en/medicines/human/referrals/adrenaline-auto-injectors [144]Medicines and Healthcare products Regulatory Agency. Adrenaline auto-injectors (AAIs). Jun 2023 [internet publication]. https://www.gov.uk/government/publications/adrenaline-auto-injectors-aais-safety-campaign/adrenaline-auto-injectors-aais Also consider needle length when choosing a suitable auto-injector.[143]European Medicines Agency. Adrenaline auto-injectors. August 2015 [internet publication]. https://www.ema.europa.eu/en/medicines/human/referrals/adrenaline-auto-injectors [144]Medicines and Healthcare products Regulatory Agency. Adrenaline auto-injectors (AAIs). Jun 2023 [internet publication]. https://www.gov.uk/government/publications/adrenaline-auto-injectors-aais-safety-campaign/adrenaline-auto-injectors-aais Prescribing an auto-injector with the correct needle length (see table below) is important to ensure that the adrenaline is injected intramuscularly rather than subcutaneously.
Ensure the patient or carer thoroughly understands how and when to use the specific device they have been prescribed as technique varies between devices.[143]European Medicines Agency. Adrenaline auto-injectors. August 2015 [internet publication]. https://www.ema.europa.eu/en/medicines/human/referrals/adrenaline-auto-injectors [144]Medicines and Healthcare products Regulatory Agency. Adrenaline auto-injectors (AAIs). Jun 2023 [internet publication]. https://www.gov.uk/government/publications/adrenaline-auto-injectors-aais-safety-campaign/adrenaline-auto-injectors-aais
Encourage people with allergies and their carers to practise the technique of using their specific adrenaline auto-injector using a trainer device. These are available free of charge from manufacturers’ websites.
Advice for patients and carers is available from the UK Medicines and Healthcare products Regulatory Agency. Adrenaline auto-injectors: advice on use Opens in new window
Auto-injectors available in the UK are Emerade®, EpiPen®, and Jext®.
Adrenaline auto-injector | Size | Length of needle (mm) |
|---|---|---|
Emerade® | Children | 16 |
Adults | 23 | |
EpiPen® | Children | 13 |
Adults | 16 | |
Jext® | Children | 13 |
Adults | 15 |
Refer patients to an age-appropriate specialist allergy service for accurate investigation, diagnosis, monitoring, ongoing management, patient education, and possible immunotherapy.[32]Resuscitation Council (UK). Emergency treatment of anaphylactic reactions: guidelines for healthcare providers. May 2021 [internet publication]. https://www.resus.org.uk/library/additional-guidance/guidance-anaphylaxis/emergency-treatment [52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134 [59]Muraro A, Worm M, Alviani C, et al. EAACI guidelines: anaphylaxis (2021 update). Allergy. 2022 Feb;77(2):357-77. https://onlinelibrary.wiley.com/doi/10.1111/all.15032 http://www.ncbi.nlm.nih.gov/pubmed/34343358?tool=bestpractice.com
Provide patients (or their parent and/or carer) after a first anaphylactic presentation with advice about:[52]National Institute for Health and Care Excellence. Anaphylaxis: assessment and referral after emergency treatment. August 2020 [internet publication]. https://www.nice.org.uk/guidance/cg134
How to recognise an anaphylactic reaction
The importance of using an adrenaline auto-injector and calling emergency services if an anaphylactic reaction occurs
How to use the adrenaline auto-injector
Biphasic reactions
How to avoid the suspected trigger (if known)
Referral to a specialist allergy service and the referral process
Patient support groups.
At subsequent presentations check that the patient understands the points above. Written information leaflets can be helpful.
Primary options
adrenaline (epinephrine): children and adults: dose depends on brand and strength of auto-injector; consult specialist or product information for guidance on dose

Choose a patient group to see our recommendations
Please note that formulations/routes and doses may differ between drug names and brands, drug formularies, or locations. Treatment recommendations are specific to patient groups. See disclaimer
Use of this content is subject to our disclaimer
