Recommendations
Urgent
Start intravenous fluids as soon as you suspect hyperosmolar hyperglycaemic state (HHS). Request immediate critical care support if you cannot get intravenous access.[6]
Give 1 L of 0.9% sodium chloride (normal saline) over 1 hour (or according to local protocols). Consider giving more rapid fluid replacement if the systolic blood pressure is <90 mmHg.[6]
In practice, seek urgent advice from the diabetes specialist team if the serum sodium on admission is very high (>150 mmol/L [>150 mEq/L]) to determine management.
Start a fixed-rate intravenous insulin infusion immediately only if there is significant ketonaemia (beta-hydroxybutyrate >1 mmol/L).[6]
Always start intravenous fluids before giving insulin. Insulin treatment prior to adequate fluid replacement may cause cardiovascular collapse.[6]
Give 0.05 units/kg/hour if blood ketones (beta-hydroxybutyrate) >1 mmol/L and ≤3.0 mmol/L and the patient is not acidotic (venous pH ≥7.3 and bicarbonate >15.0 mmol/L), or according to local protocols.
Give 0.1 units/kg/hour if blood ketones (beta-hydroxybutyrate) >3.0 mmol/L or ketonuria (2+ or more) with a pH <7.3 and bicarbonate <15 mmol/L (i.e. mixed diabetic ketoacidosis and HHS), or according to local protocols.
Identify and treat any underlying cause.[6]
Common causes include myocardial infarction, sepsis, and stroke.[9]
Involve senior or critical care support if:[6]
Serum osmolality is >350 mOsm/kg (>350 mmol/kg) [ Osmolality Estimator (serum) Opens in new window ]
Serum sodium is >160 mmol/L (>160 mEq/L)
Venous/arterial pH is <7.1
Serum potassium is <3.5 mmol/L (<3.5 mEq/L) or >6 mmol/L (>6 mEq/L) on admission
Glasgow Coma Scale (GCS) score is <12 or AVPU (Alert, Voice, Pain, Unresponsive) scale score is abnormal [ Glasgow Coma Scale Opens in new window ]
Oxygen saturation is <92% on air (assuming normal baseline respiratory function)
Systolic blood pressure is <90 mmHg
Pulse is >100 or <60 bpm
Urine output is <0.5 mL/kg/hour
Serum creatinine is >200 micromol/L (>2.3 mg/dL)
The patient is hypothermic
The patient has a concurrent macrovascular event such as myocardial infarction or stroke, or other significant comorbidity.
In practice, heart failure and significant renal impairment (chronic kidney disease and/or acute kidney injury, particularly if eGFR <30 mL/minute/1.73 m²) should also warrant senior or critical care support.
Be aware that some patients may present with mixed HHS and diabetic ketoacidosis (DKA).[2][6] These patients require modification of treatment, which is not covered in this topic, though treatment with fluids and insulin should be started without delay.
Key Recommendations
The key management of HHS should include:[6]
Fluid replacement and fixed-rate intravenous insulin
Correction of serum osmolality, electrolytes, and blood glucose
Prevention of venous thromboembolism, complications of treatment, and foot ulceration
Treatment of the underlying cause.
Always maintain an accurate fluid balance chart.[6]
Involve the diabetes specialist team early and ensure all patients are reviewed by a senior colleague within 1 hour of presentation.[6]
Before discharge, most patients should be switched to subcutaneous insulin, according to advice from the diabetes specialist team.[6] All patients should have appropriate diabetes education and follow-up with the diabetes specialist team.[6]
Treatment should aim to:[6]
Correct the serum osmolality, electrolytes, and blood glucose:
Aim for a gradual decline in serum osmolality (3-8 mOsm/kg/hour [3-8 mmol/kg/hour])
Avoid rapid correction of blood glucose and sodium; determine intravenous fluid management based on the patient’s initial sodium level
Sodium should not change by more than 10 mmol/L (10 mEq/L) in 24 hours
Blood glucose should fall at a rate between 4.0 mmol/L/hour (72 mg/dL/hour) and 6.0 mmol/L/hour (108 mg/dL/hour)
Avoid hypoglycaemia; aim for an initial target blood glucose of 10 to 15 mmol/L (180-270 mg/dL) until the patient is eating and drinking normally, after which an individual target glucose should be set by the diabetes specialist team and the patient.
Replace fluid to maintain an adequate fluid balance in order to achieve:
A minimum urine output of 0.5 mL/kg/hour
A positive fluid balance of 2 to 3 L by 6 hours, 3 to 6 L by 12 hours, and replacement of the remaining fluid losses at 12 to 24 hours
Replacement of estimated fluid losses within 24 hours. This is dependent on factors such as initial degree of dehydration, body weight, and response to treatment
Prevent arterial or venous thrombosis, complications of treatment, and foot ulceration.
Assess the patient for complications of treatment every 1 to 2 hours. These include cerebral oedema and central pontine myelinolysis (look for a deteriorating conscious level) as well as fluid overload.
Seek advice from a senior colleague if the patient is failing to improve at any point.
Be aware that some patients may present with mixed HHS and DKA.[2][6]
Start intravenous fluids as soon as you suspect HHS. Request immediate critical care support if you cannot get intravenous access.[6]
Give 1 L of 0.9% sodium chloride (normal saline) over 1 hour (or according to local protocols). Consider giving more rapid fluid replacement if the systolic blood pressure is <90 mmHg.[6]
In practice, seek urgent advice from the diabetes specialist team if the serum sodium on admission is very high (>150 mmol/L [>150 mEq/L]) to determine management.
Practical tip
Determine the initial rate of intravenous fluid by assessing the degree of initial dehydration and any coexisting comorbidities. Maintain caution, particularly with older patients, because giving intravenous fluids too quickly may precipitate heart failure, but giving them too slowly may fail to reverse acute kidney injury.[6]
Typical fluid losses in HHS may be 100 to 220 mL/kg (10-22 L in a person weighing 100 kg).[6]
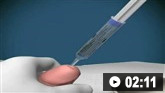
How to insert a peripheral venous cannula into the dorsum of the hand.
Start a fixed-rate intravenous insulin infusion (FRIII) if there is significant ketonaemia (beta-hydroxybutyrate >1 mmol/L).[6]
Always start intravenous fluids before giving insulin. Insulin treatment prior to adequate fluid replacement may cause cardiovascular collapse.[6]
Use a rate of 0.05 units/kg/hour if blood ketones (beta-hydroxybutyrate) are ≤3.0 mmol/L and the patient is not acidotic (venous pH ≥7.3 and bicarbonate ≥15.0 mmol/L), or according to local protocols.[6]
Use a rate of 0.1 units/kg/hour if the patient is acidotic (pH <7.3 and bicarbonate <15 mmol/L) and blood ketones (beta-hydroxybutyrate) are >3.0 mmol/L or ketonuria (2+ or more), or according to local protocols (i.e. if the patient has mixed DKA and HHS).[6]
Evidence: Insulin timing
Guidelines differ in their recommendations for when to start insulin for people with HHS. This has not been formally studied and therefore is based on consensus only.
Guidelines agree that the initial management of HHS should focus on intravenous fluid resuscitation, which will also have the effect of reducing blood glucose levels. However, there is no published evidence on when insulin treatment should be started, therefore the recommendations are by consensus and vary between different guidelines.[1][6][17]
A before-and-after study (n=201, published 2019) of adult patients with hyperglycaemic crisis (DKA or HHS) supports the use of less-intensive insulin regimens as recommended in the guidelines.[64]
The intensive insulin therapy consisted of bolus doses of insulin, target blood glucose of 5.6 to 11.1 mmol/L (100-200 mg/dL), and faster insulin intravenous infusion rates. The less-intensive protocol had no insulin bolus, target blood glucose of 11.1 and 16.7 mmol/L (200-300 mg/dL), and a slower insulin intravenous infusion rate.
Patients treated with high-intensity insulin therapy had a longer hospital stay (mean stay 150 hours vs.114 hours), longer intensive care unit stay (mean stay 30 hours vs. 19 hours), and higher prevalence of hypoglycaemic events (35% vs. 1%).
There was a further suggestion of harm with five deaths in the intensive insulin therapy group but only one with less-intensive therapy.
Start a fluid balance chart.[6]
Insert a urinary catheter to monitor urine output (aim for 0.5 mL/kg/hour).[6]
How to insert a urethral catheter in a male patient using sterile technique.
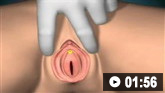
How to insert a urethral catheter in a female patient using sterile technique.
Identify and treat any precipitating acute illness.[6]
Common causes include myocardial infarction, sepsis, and stroke.[9]
Evidence: Precipitating causes
HHS most commonly occurs in older people with type 2 diabetes and comorbidities. Infection is the most common precipitating factor.
People with HHS are generally older than people with DKA.[9]
A retrospective chart review study found patients with diabetic acidosis were younger (n=134, mean age 33 years) while patients with HHS were significantly older (n=278, mean age 63 years).[48]
However, HHS does also occur in young adults and even children.[13][49][50]
Infection represents the commonest precipitating cause of HHS with the most common being pneumonia (40% to 60%) and urinary tract infection (5% to 16%).[3][9][17]
Other precipitating factors of HHS include:
Severe dehydration caused by underlying medical illness (e.g., stroke, myocardial infarction, trauma) due to the release of counterregulatory hormones and/or reduced water intake[1][9][11]
Medications including glucocorticoids, thiazide diuretics, phenytoin, beta-blockers, and atypical antipsychotics[9][24]
Poor compliance with insulin treatment and previously undiagnosed diabetes.[25][48]
One study looking at predisposing factors compared 135 people with HHS with 135 age-matched controls with diabetes. The authors did a multivariate analysis and found female sex, newly diagnosed diabetes, and acute infection to be the only three independent predictors of HHS.[51]
Establish a monitoring regimen for your patient according to your local protocol. The Joint British Diabetes Societies (JBDS) guideline recommends:[6]
Hourly blood glucose, sodium, potassium, urea, and calculated serum osmolality for the first 6 hours; sodium and osmolality monitoring can be reduced to every 2 hours after 6 hours if serum osmolality is decreasing by 3 to 8 mOsm/kg/hour (3-8 mmol/kg/hour) [ Osmolality Estimator (serum) Opens in new window ]
Continuous pulse oximetry[6]
Continuous cardiac monitoring if necessary[6]
Vital signs including early warning score (EWS).
Practical tip
In practice, local recommendations for monitoring may vary and measurement of serum osmolality to monitor the patient's response to treatment may not be commonly used; check your local protocol. As an alternative:
Reduce monitoring of blood glucose to every 2 hours after 6 hours if the blood glucose is stable (around 12-15 mmol/L [216-270 mg/dL]).
Reduce monitoring of sodium, potassium, and urea to every 2 hours after 6 hours if these are improving.
Start prophylactic low molecular weight heparin, unless it is contraindicated.[6] See Venous thromboembolism (VTE) prophylaxis.
Ensure all patients are reviewed by a senior colleague within 1 hour of presentation and are referred to the diabetes specialist team.[6]
Check that the patient’s heels and other pressure areas are protected and that daily foot checks are in place.[6]
Continue intravenous fluids. Adjust the rate and type of intravenous fluid according to the degree of dehydration, fluid balance, risk of heart failure, and monitoring of glucose, urea, electrolytes, and serum osmolality (monitor these hourly for the first 6 hours). The rate of intravenous fluids should usually be 0.5 to 1 L/hour. The Joint British Diabetes Societies (JBDS) guideline recommends the following:[6]
Consider continuing 0.9% sodium chloride (normal saline) at the same rate if:[6]
Sodium is increasing
AND
Serum osmolality is decreasing by 3 to 8 mOsm/kg/hour (3-8 mmol/kg/hour)
Consider increasing the rate of 0.9% sodium chloride if:[6]
Sodium is increasing
AND
Serum osmolality is increasing (or declining at less than 3 mOsm/kg/hour [<3 mmol/kg/hour])
AND
Positive fluid balance is inadequate
OR
Blood glucose is decreasing by <5 mmol/L/hour (<90 mg/dL/hour)
AND
Positive fluid balance is inadequate
Consider switching to 0.45% sodium chloride and continuing this at the same rate if:[6]
Serum osmolality is increasing
AND
Positive fluid balance is adequate
Aim to keep blood glucose 10-15 mmol/L (180-270 mg/dL) in the first 24 hours.[6]
If blood glucose falls below 14 mmol/L (<252 mg/dL), start 5% or 10% glucose at 125 mL/hour and continue 0.9% sodium chloride solution.[6]
Practical tip
In practice, measurement of serum osmolality to monitor the patient's response to treatment may not be commonly used; check your local protocol and seek senior advice if you are unsure about the patient’s fluid requirements.
Practical tip
Be aware that hypotonic (0.45% sodium chloride) solutions may be appropriate for people with HHS and significant hypernatraemia. However, you should only give hypotonic solutions to prevent a rise in sodium level if serum osmolality is not declining concurrently.[6]
A rise in sodium level is inevitable because fluid replacement (without insulin) will lower blood glucose which will reduce osmolality causing a shift of water into the intracellular space.[6]
In practice, seek urgent advice from the diabetes specialist team if the serum sodium on admission is very high (>150 mmol/L [>150 mEq/L]) to determine management.
If blood glucose is decreasing by <5 mmol/L/hour (<90 mg/dL/hour) and positive fluid balance is adequate:[6]
Start a fixed-rate intravenous insulin infusion (FRIII) at a rate of 0.05 units/kg/hour (or according to local protocols) if this has not been started already.
If an FRIII has been started already, increase the rate to 0.1 units/kg/hour (or according to local protocols).
Maintain serum potassium level within the normal range using premixed 0.9% sodium chloride with potassium chloride. Replace as follows:[6]
Serum potassium level | Potassium replacement |
|---|---|
<3.5 mmol/L (<3.5 mEq/L) | Involve senior or critical care support as additional potassium needs to be given |
3.5 to 5.5 mmol/L (3.5 to 5.5 mEq/L) | 40 mmol/L of infusion solution |
>5.5 mmol/L (>5.5 mEq/L) | None |
Practical tip
Use 0.9% sodium chloride with pre-mixed potassium chloride as the default fluid for resuscitation in HHS.
Manually adding potassium to intravenous fluids in general clinical areas is unsafe as this can result in accidental overdose of potassium, which can be fatal.
Ensure the patient has ongoing monitoring; this should include:
The Joint British Diabetes Societies (JBDS) guideline recommends continued hourly blood glucose monitoring; of sodium, potassium, urea, and serum osmolality can be reduced from hourly to every 2 hours (as long as serum osmolality is decreasing by 3-8 mOsm/kg/hour [3-8 mmol/kg/hour]).[6]
Adjust the rate of intravenous fluids and potassium replacement according to these results. See section on 1 to 6 hours above.
Maintain an accurate fluid balance chart.
Practical tip
In practice, local recommendations for monitoring may vary and measurement of serum osmolality to monitor the patient's response to treatment may not be commonly used; check your local protocol. As an alternative:
Reduce monitoring of blood glucose to every 2 hours after 6 hours if the blood glucose is stable (around 12-15 mmol/L [216-270 mg/dL])
Reduce monitoring of sodium, potassium, and urea to every 2 hours after 6 hours if these are improving.
Continue fixed-rate intravenous insulin infusion (FRIII).[6]
Maintain blood glucose in the range 10 to 15 mmol/L (180-270 mg/dL).[6]
Continue treatment of any underlying cause. Seek advice from a senior colleague if the patient is not improving clinically.[6]
Assess the patient for complications of treatment every 1 to 2 hours. These include cerebral oedema and central pontine myelinolysis (look for a deteriorating conscious level) as well as fluid overload.[6]
If you suspect cerebral oedema or central pontine myelinolysis, seek immediate senior and critical care support.
Consider ordering a CT head if the GCS score is deteriorating or the patient has a new or worsening headache.[67]
Ensure that the patient has been referred to the diabetes specialist team.[6]
Continue to monitor blood glucose hourly. Reduce monitoring of sodium, and serum osmolality to every 4 hours if these are improving.[6]
Adjust the rate of intravenous fluids and potassium replacement according to these results. See section on 1 to 6 hours above.
Maintain an accurate fluid balance chart.
Practical tip
Although sodium and serum osmolality should continue to improve, these are unlikely to have normalised by 24 hours; complete normalisation may take up to 72 hours.[6]
Continue fixed-rate intravenous insulin infusion (FRIII).[6]
Increase or decrease the rate of FRIII by 1 unit/hour (or according to local protocols) maintain blood glucose in the range 10 to 15 mmol/L (180-270 mg/dL).[6]
Continue to monitor for complications of treatment.[6]
Continue treatment of any underlying cause. Seek advice from a senior colleague if the patient is not improving clinically.[6]
Ensure the patient is recovering, starting to eat and drink, and biochemical and clinical markers have returned to normal or baseline (pre-acute episode) levels.[6]
Assess for ongoing signs of the underlying cause and complications of treatment.[6]
Continue intravenous fluids until the patient is eating and drinking normally. Stop the fluid balance chart once intravenous fluids are no longer required.
Give a variable rate intravenous insulin infusion if the patient is not eating and drinking.
Make sure the patient has been reviewed by the diabetes specialist team; they will convert the patient to a subcutaneous insulin regimen when biochemically stable.
In addition, ensure the patient has:[6]
Daily foot checks
Daily urea and electrolytes
Low molecular weight heparin continued until the day of discharge (consider extended treatment for high-risk patients). See Venous thromboembolism (VTE) prophylaxis.
Early mobilisation
Catheter removal when clinically appropriate.
Ensure the patient has follow-up with the diabetes specialist team and appropriate diabetes education.[6]
Use of this content is subject to our disclaimer