Introduction
Anterior knee pain or “runner’s knee” is a common presenting complaint in the MSK clinic. In active individuals, progressive overload of the patellofemoral joint, and disruption of the under surface of the hyaline cartilage of the kneecap, can cause significant disability, pain and impact training. We discuss the key risk factors for chondromalacia, the sports and biomechanical risk factors, imaging findings and the latest cartilage therapies to preserve and treat this condition.
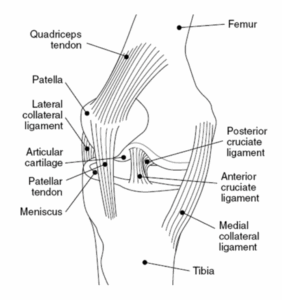
The knee consists of the patellofemoral joint and medial and lateral tibiofemoral joint. Though chondromalacia can also occur in joints such as the hip and shoulder, damage to the cartilage located on the posterior aspect of the patella that articulates with the trochlear groove of the femur, is the most common manifestation of the condition in MSK clinics (1).
Figure 1: Diagram of the knee joint
Chondromalacia patella is a radiological finding (2). It sits on a spectrum from normal physiological loading to positions where the kneecap is unstable or misaligned. The challenge for MSK clinicians is to work up the patient, understand why the radiological changes have occurred and treat the reversible biomechanical, structural and ortho biological risk factors.
Presentation pattern
Patients usually present with symptoms after undertaking exercises that increase joint loading forces at the patella. These typically include ascending or descending stairs, squatting or running that cause (1, 3):
- Anterior knee pain
- Gradual onset of vague, non-specific retro patellar or prepatellar pain
- Retro patellar crepitus, effusion or wasting of quadriceps (4)
It is important to remember that chondromalacia patella rarely occurs by itself and sits along a spectrum of other tendon and soft tissue conditions that cause patellofemoral pain syndrome (PFPS) or “runner’s knee” (5).
Who is affected?
- Women are disproportionately affected: The Q angle is the angle formed between the lateral line of the pull of the quadriceps and the patella, measured from the centre of the anterior superior iliac spine (ASIS) to the tibial tuberosity. The average Q angle for men is 14 degrees, and 17 in women, due to a broader pelvis. A higher mean Q angle value is said to contribute to patellofemoral pain (6).
- Active young adults: Those engaged in running sports or individuals who frequently subject their patellofemoral joints to stress through repetitive stair climbing or kneeling are at a higher risk for developing chondromalacia.
Figure 2: Diagrammatic representation of the Q angle with respect to the patella and ASIS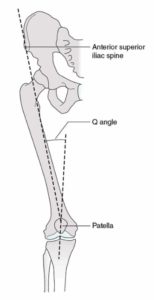
Diagnostic difficulties
- Determining the exact prevalence of chondromalacia is challenging due to its overlap with patellofemoral joint syndrome, variations in diagnostic criteria, and that it is rarely seen alone without other pathology.
- A recent systematic review estimated that the annual prevalence of patellofemoral pain in the general population is approximately 22.7%, rising to 28.9% among adolescents (7).
- In the general population, the prevalence of chondromalacia can be as high as 36.2%, with rates reaching up to 50% in middle-aged individuals between 30 and 40 years of age (8).
Figure 3: Risk factors associated with chondromalacia patella

Risk factors
Table 1: Risk factors for chondromalacia patella
| Category | Risk Factor | Description |
| Intrinsic Factors | Biomechanical Alignment Issues | |
| Patellar Malalignment | Misalignment of the patella in the femoral groove increasing stress on the cartilage | |
| Q-Angle | An increased Q-angle predisposing individuals to patellar tracking problems | |
| Rotational malalignment of lower limbs | Conditions leading to improper alignment and movement patterns, putting extra stress on the knee joint – including femoral anteversion or tibial torsion, flat feet or overpronation | |
| Valgus alignment | The misalignment shifts load disproportionately onto certain areas of the knee cartilage, increasing wear and tear. Over time, this uneven stress can lead to softening and breakdown of the cartilage, as the patella may not track smoothly | |
| Rotational malrotation of lower limbs | Excessive internal or external rotation of the femur or tibia disrupts normal knee mechanics and patellar tracking, causing the patella to move unevenly within the trochlear groove. | |
| Trochlear dysplasia | Causes patellar instability. This instability leads to uneven pressure, abnormal shear forces, and repeated trauma on the cartilage, wearing it down over time. | |
| Muscle Imbalances | ||
| Gluteal Weakness | Predisposes to relying excessively on the tensor fasciae late (TFL) which pulls on the iliotibial band (ITB) causing lateral maltracking of the patella | |
| Quadriceps Weakness | Weakness, particularly in the vastus medialis obliquus (VMO), resulting in poor patellar tracking | |
| Tight Hamstrings or Iliotibial (IT) Band | Tightness in these areas altering knee mechanics and increasing patellofemoral joint stress | |
| Previous Knee Injuries | ||
| History of Knee Injuries | Injuries such as fractures, ligament tears, or dislocations contributing to abnormal patellar movement | |
| Age and Gender | ||
| Adolescents and Young Adults | More commonly affected, particularly those active in sports | |
| Females | Higher risk due to wider pelvis and larger Q-angle | |
| Extrinsic Factors | Physical Activity | |
| Repetitive Stress | High-impact sports or activities involving repetitive knee bending | |
| Sudden Increases in Activity Level | Abrupt changes in exercise intensity or duration stressing the knee joint excessively | |
| Footwear | ||
| Improper Shoes | Wearing shoes that do not provide adequate support or are unsuitable for the specific sport | |
| Training Errors | ||
| Poor Technique | Incorrect form or technique during physical activities increasing the risk | |
| Inadequate Warm-Up or Cool-Down | Skipping warm-up or cool-down exercises making muscles and joints more susceptible to injury | |
| Environmental Factors | ||
| Surface Type | Running or exercising on hard or uneven surfaces increasing the impact on the knees and contributing to cartilage wear |
What causes Chondromalacia Patella?
- Overuse: Repeated stress on the knee joint, often due to high-impact sports or activities that involve frequent knee bending, such as running, jumping, or cycling.
- Injury: Direct trauma to the knee, such as a blow or fall, can damage the patellar cartilage.
- Malalignment: Anatomical abnormalities like patellar maltracking, where the patella does not move smoothly within the trochlear groove, can cause uneven pressure and wear on the cartilage.
- Muscle Imbalance: Weakness or imbalance in the muscles around the knee, particularly the quadriceps, can alter patella’s tracking and increase stress on specific areas of cartilage.
- Degeneration: Age-related wear and tissue senescence can lead to breakdown of cartilage.
Figure 4: Theories on the development of chondromalacia patella
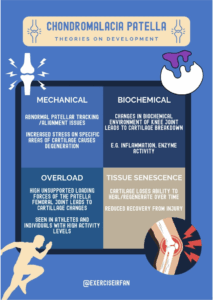
Theories on Development and Who Is Affected
- Mechanical Theory: Suggests that abnormal patellar tracking or alignment issues lead to increased stress on specific areas of the cartilage, causing degeneration.
- Biochemical Theory: Proposes that changes in the biochemical environment of the knee joint, such as inflammation or enzyme activity, contribute to cartilage breakdown.
- Overload Theory: Emphasises repetitive overloading of the patellofemoral joint, often seen in athletes or individuals with high physical activity levels.
- Tissue senescence: over time cartilage tissue loses its ability to regenerate/ generate healthy tissue and recover to injury
Chondromalacia patella is common among young athletes, particularly runners, cyclists, and those involved in sports requiring repetitive knee movements. However, it can also affect older adults due to degenerative changes in the cartilage.
Figure 5: Overview of forces through the patellofemoral joint

Forces in the Patellofemoral Joint
To treat chondromalacia patella effectively, the clinician must understand the contributing forces at play in the joint.
Figure 6: Diagrammatic representation of forces through the patellofemoral joint
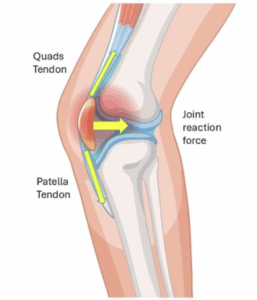
The patella has the quadricep attachment superiorly and the patellar tendon attachment inferiorly. As the tibiofemoral (knee) joint is flexed, the pulling forces on either end of the patella increases. This in turn increases the patellofemoral joint reaction force (yellow arrow). This force increases with knee flexion angles. For example, at 90° of knee flexion, the patellofemoral joint reaction force can be several times body weight.
In cases of chondromalacia patella, this high compressive force leads to greater friction and pressure on the damaged cartilage, accelerating cartilage wear and pain.
A systematic review by Harvi F Hart et al. (2022) showed that activities with greater knee flexion (e.g., squats, stairs) generate higher patellofemoral joint reaction forces (PFJR) than those with smaller knee flexion (e.g., walking) (9).
PFJRF in Everyday Activities: Average peak PFJRFs in healthy individuals are:
- Walking: 0.9×BW
- Stair ascent: 3.2×BW
- Stair descent: 2.8×BW
- Running: 5.2×BW
PFJRF in Therapeutic Exercises: Ranges widely, from 1 to 18×BW, depending on exercise type and variation:
- Squats: 1–18×BW
- Lunges: 3–6×BW
- Cycling: 1–7×BW
- Jumping: 9–11×BW
Exercise Variations Matter: Specific variations increase PFJRF, e.g., lunges with strides vs. without, or squats with knees beyond toes vs. behind toes.
Quadriceps Force:
- The quadriceps muscle plays a major role in PFJ mechanics. When the quadriceps contract, they generate force across the knee joint that increases PFJ compression.
Patellofemoral Joint Reaction Force:
- This force increases with greater degrees of knee flexion. For example, at 90° of knee flexion, the patellofemoral joint reaction force can be several times body weight.
- In cases of chondromalacia patella, this high compressive force leads to greater friction and pressure on the damaged cartilage, accelerating cartilage wear and pain.
- A low lying patella (Patella Baja) – is also associated with higher PFJ forces.
Contact Area and Pressure:
- As the knee flexes, the contact area between the patella and the femur increases, distributing forces over a larger surface area.
- High localised stresses can contribute to cartilage breakdown in dysplasia, or where there are cartilage defects.
Lateral Tracking Forces:
- Abnormal tracking of the patella: laterally, or higher (patella alta) can cause abnormal loading, and risk instability
Chondromalacia and the soft tissue structures of the knee
- Hoffa’s Fat Pad: Inflammation or impingement
- Chondral Surfaces: injury to the cartilage
- Synovium: Inflammation of swelling
- Patella and Trochlea Groove: Bone marrow oedema. Osteochondral defects & degenerative changes.
- Patella tendinitis: inflammation and pain at the patellar insertion of the tendon
Working up Chondromalacia Patellae (CP) – MSK imaging
- Magnetic Resonance Imaging (MRI) is the most used non-invasive imaging technique for diagnosing chondromalacia patellae (CP).
Table 2: The Modified Outerbridge Criteria Grading System (Grade 0-4)
| Grade | Description | Main clinical features |
| Grade 0 | Normal cartilage present | Healthy cartilage with smooth and intact features |
| Grade 1 | Intact surface of the articular cartilage compared with the surrounding normal cartilage.
Findings: Inhomogenous; high signal; surface intact; cartilage swelling |
Early, mild anterior knee pain.
May have occasional crepitus or discomfort during activities like stair climbing or squatting. |
| Grade 2 | Partial thickness defect of the cartilage.
Findings: Superficial ulceration, fissuring, fibrillation; involves <50% of cartilage thickness |
Increased pain with physical activity.
Crepitus may be more pronounced. Localised swelling or tenderness around the patella. |
| Grade 3 | Fissuring of the cartilage to the level of the subchondral bone.
Findings: Ulceration fissuring, fibrillation; includes >50% of depth of cartilage |
Persistent pain, even during rest or minimal activity.
Swelling and reduced range of motion. Crepitus and grinding sensations may worsen. |
| Grade 4 | Exposed subchondral bone with full thickness chondral wear | Severe, constant anterior knee pain.
Joint instability and functional impairment. Swelling, locking, or giving way of the knee. |
MRI Images
Figure 7a: 43 Year old with patellofemoral joint pain. There is moderate secondary osteoarthritis of the patellofemoral joint secondary to patella maltracking, patella alta, trochlear dysplasia. There is grade IV chondromalacia along the medial and lateral patellofemoral joints (arrow). Axial PD/FS sequence.
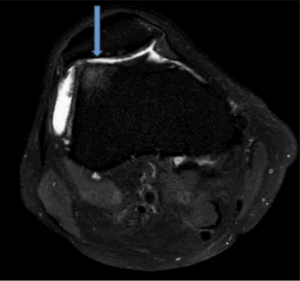
Figure 7b: Demonstrates patella alta with oedematous signal in the superior lateral portion of Hoffas fat, consistent with Hoffas fat pad entrapment. Sagittal view (PD /FS)
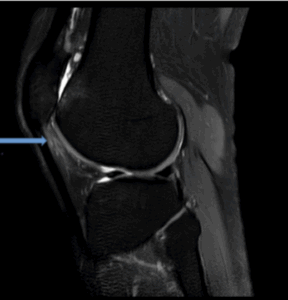
Figure 7c: Demonstrates patella alta with complete chondral surface loss along the lateral patella and trochlea (grade IV). Associated subchondral cysts along the lateral trochlea. Sagittal view PD sequence.
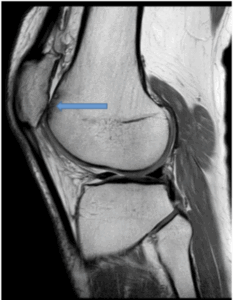
Figure 8a: 17 year old demonstrates kissing contusion pattern from recent patella dislocation. Kissing contusion describes the contusion along the medial patella facet and lateral femoral condyle (blue-yellow arrow). Associated full thickness tearing of the insertion of the medial patellofemoral ligament complex (green arrow). Axial PD/FS sequence
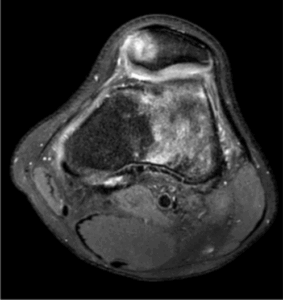
Figure 8b: 17 year old coronal (PD/FS) demonstrates tear of the patella insertion of the medial patellofemoral ligament complex (blue arrow). Small minimally displaced osseous fragment along the inferior medial patella (green arrow).
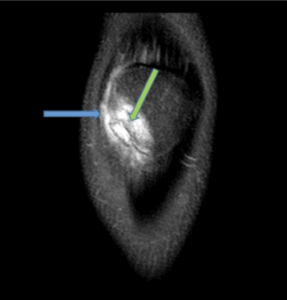
Figure 9a: 15 year old patient with patella maltracking and a history of recurrent patella subluxation. The patient has trochlear dysplasia (green arrow). There is majority chondral surface delamination of along the medial patella facet (grade III, blue arrow, axial view, PD/FS).
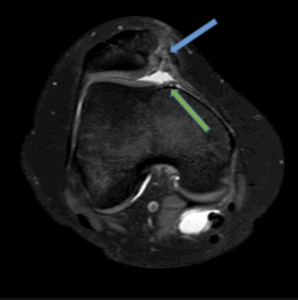
Figure 9b: Patella maltracking with patella alta and oedema in the superior lateral aspect of Hoffas fat pad, consistent with Hoffas fat pad entrapment (blue arrow, sagittal view, PD/FS).
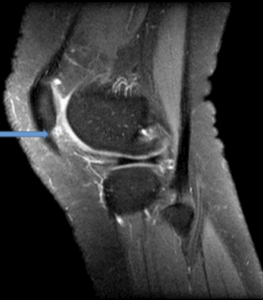
Figure 10a: 16 Year old history of twisting injury. Features show recent patella dislocation with kissing contusion pattern and effusion of the suprapatella bursa. The patella remains partially subluxed with moderate trochlear dysplasia (red arrow) and full thickness chondral surface loss along the lateral patella facet (grade IV, blue arrow).
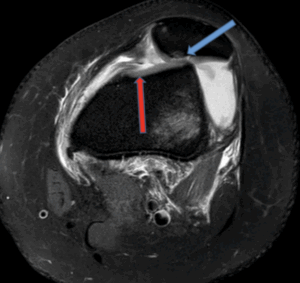
Figure 10b: Osteochondral defect secondary to patella dislocation with kissing contusion pattern. (Sagittal PD)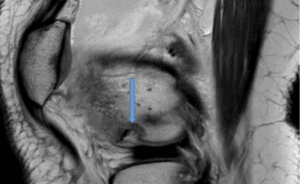
Figure 11: Significance of clinical tests in diagnosing chondromalacia patella

Our systematic review looked at the sensitivity and specificity of different MRI techniques and specific imaging findings in comparison to clinical diagnostic tools. The takeaway points include:
- The use of MRI to observe changes in knee structures, especially fat tissue thickness, can provide a reasonably accurate diagnosis
- Inversion Recovery–Fast Spin-Echo (IR-FSE) Imaging when used in grading advanced disease, MR Arthrography and Intermediate Weighted-Fat Suppressed Fast Spin Echo Image (Iw-FS-FSE) are all techniques which offered both sensitivities and specificities greater than 80%
- The Patellar Sliding Test (PST) offered the highest sensitivity and specificity among clinical tests
- The VISA-P was the most specific (80.8%) out of the diagnostic questionnaires used to diagnose CP
Table 4: Sensitivity and specificity of diagnostic tests in diagnosing chondromalacia patella
| Imaging Findings | Sensitivity | Specificity |
| Imaging techniques | ||
| MR Arthrography (11) | 80 | 98 |
| CT Arthrography (11) | 73 | 100 |
| T2-weighted (11) | 47 | 91 |
| Proton Density (11, 12) | 13 – 76 | 93 – 99 |
| Water Selective Cartilage Scan (WATS-c) (12) | 54-67 | 100 |
| Water Selective Fluid Scan (WATS-f) (12) | 50-58 | 99-100 |
| Intermediate Weighted-Fat Suppressed Fast Spin Echo Image (Iw-FS-FSE) (12) | 88-94 | 97-98 |
| Inversion Recovery–Fast Spin-Echo (IR-FSE) Imaging – grading early disease (13) | 75 | 94 |
| Inversion Recovery–Fast Spin-Echo (IR-FSE) Imaging – grading advanced disease (13) | 80 | 99 |
| SPIR sequence (14) | 89 | |
| MTC sequence (14) | 94 | |
| T2 mapping (15) | 61 | 64 |
| Fat saturated (16) | 92 | |
| Fat suppressed (17, 18) | 66-72 | 88 |
| Specific findings in knee | ||
| Patellar Cartilage Cross-Sectional Area (PCCSA) – 116.24 mm (11, 19) | 72 | 72 |
| Prepatellar subcutaneous fat tissue thickness (PSFTT) >5.80 mm (20) | 80 | 82.9 |
| Medial subcutaneous fat tissue thickness (MSFTT)
> 25.615 mm (20) |
84.4 | 75.7 |
| Clinical tests | ||
| Patellar Sliding Test (PST) (21) | 89 | 85 |
| Clarke’s Test (22) | 39 | 67 |
| Anterior Patella discomfort and pain (18) | 28 | |
| Pain after walking stairs and prolonged sitting (without findings in clinical examination) (18) | 32 | |
| Kujala Patellofemoral Score (KPS) cut off score of 71 (23) | 78.6 | 69.2 |
| Knee injury and Osteoarthritis Outcome Score for Patellofemoral pain and osteoarthritis (KOOS-PF) cut off score of 53.4 (23) | 78.6 | 73.1 |
| Victorian Institute of Sports Assessment for Patellar tendons questionnaire (VISA-P) cut off score of 48.5 (23) | 78.6 | 80.8 |
Figure 12: Management strategies for chondromalacia patella
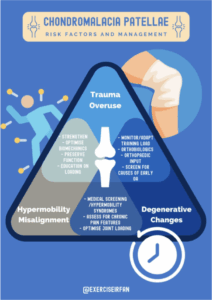
Management
Initial treatment should involve active therapies, that optimise joint loading, and biomechanics before interventions.
Conservative management
- Rest
- Activity modification
- Rehabilitation
- Nonsteroidal anti-inflammatory medication, which is proven more effective than steroids
Rehabilitation should include physiotherapy focusing on:
- Closed chain short arc quadriceps exercises
- Specific strengthening of the vastus medialis obliquus
- Core muscle strengthening
- Strengthening of hip external rotators
- Quadriceps muscle strengthening through various exercises significantly reduces anterior knee pain in early cases of CMP.
Injection therapy
If conservative management as above has minimal benefit, there are several types of non-surgical intervention that can be explored to help alleviate the symptoms.
The use of injection therapy should not be used as a sole treatment for chondromalacia patellae. While injections can help reduce symptoms, they do little to address the underlying biomechanical issues causing pain and dysfunction. Injections are best utilised as part of a multimodal treatment plan that includes exercise, physical therapy, and activity modification.
1. Corticosteroid injections
Corticosteroid injections have traditionally been a popular treatment option for their anti-inflammatory and pain-relieving properties. They can reduce short term pain symptoms, to aid rehab. Injecting clinicians should carefully monitor the total steroid burden and be mindful of the frequency of injections to avoid harm (chondrotoxicity)
- Pain relief: Corticosteroid injections provide significant effective short-term pain relief, however the effects of this tend to wear out within 3-6 months
- Function: Patients may see functional improvement with increased range of motion however, without addressing underlying mechanical issues, pain and functional gain will likely be lost
They are best utilised as part of a comprehensive treatment plan that includes physical therapy and activity modification (24).
2. Hyaluronic acid (viscosupplementation) injections
Hyaluronic acid injections aim to restore the viscoelastic properties of synovial fluid, enhancing joint lubrication and cushioning. Studies suggest hyaluronic acid injections may provide anti-inflammatory, analgesic, and chondroprotective benefits. This injection should be considered on a case-by-case basis.
- Hyaluronic acid is shown to provide anti-inflammatory, analgesic and chondroprotective action to the patellofemoral joint
- A prospective randomised study by Maia et al. (2019) on the use of viscosupplementation for knee osteoarthritis pain showed improved pain, stiffness and function for up to 6 months (25).
- A study by Shuai Zhang et al. (2019) showed that hyaluronic acid injection produces better long-term efficacy for treatment of early chondromalacia patella rather than advanced stages (26).
- A recent Korean study by Park JG et al found that intra-articular hyaluronic acid injections were associated with significant delay in total knee arthroplasty among patients with knee osteoarthritis. This finding supports the hypothesis that hyaluronic acid injections can not only improve symptoms but also protect cartilage and prevent further cartilage degradation (27).
Hyaluronic acid injections offer moderate symptom relief for chondromalacia patella. If there is underlying early OA within the knee, it may be considered as non-steroid based option.
3. Platelet rich plasma (PRP) injections
PRP therapy involves injecting a concentration of the patient’s own platelets to promote healing and reduce inflammation. These may be effective when there is associated tendon pathology (tendinopathy).
- Recent systematic reviews and meta-analyses suggest that platelet rich plasma injections outperform corticosteroids, viscosupplementation, and placebo when it comes to symptomatic knee osteoarthritis. However, there is still a lack of data behind the use of PRP injections specifically for patellofemoral pain syndrome and chondromalacia patella (28, 29).
- A study by Li M et al (2011) showed intra-articular PRP injections are a safe treatment for knee cartilage degeneration, reducing pain and swelling while improving quality of life, though further large-scale, long-term studies are needed to validate their efficacy and safety (30).
- A cohort study by Ostojic M et al (2024) comparing patients with chondromalacia patella being treated with PRP vs physiotherapy only showed a statistically significant difference favouring PRP over a 3-to-6-month period (31).
PRP injections appear to be a promising regenerative treatment for both pain relief and functional improvement, when tendon pathology (tendinopathy) or OA is present with underlying joint. More high-quality, large-scale studies are needed to fully validate their efficacy and long-term benefits however they offer a non-steroid based option for treatment.
4. Stem cell therapies
Stem cell therapy aims to regenerate damaged cartilage by injecting stem cells capable of differentiating into chondrocytes (cartilage cells).
- The potential for cartilage repair and regeneration addresses one of the underlying causes of chondromalacia patella
- A study by Pak J et al (2013) looked at the use of adipose-tissue-derived stem cells for CP. In three months, patients experienced an 80-90% improvement in pain which persisted for over 1 year (32).
- Repeat MRI scans in 3 months showed considerable improvement of damaged tissues
Stem cell therapy is an emerging and experimental treatment for chondromalacia patella with high potential for addressing underlying cartilage damage. However, extensive research and long-term studies are required to establish its safety, efficacy, and standardised protocols.
In summary, intra-articular injections can be effective for both pain relief and functionality in the short-term. However, without addressing underlying mechanical and structural abnormalities, pain is likely to re-present within a year.
Surgical management
Surgical management for chondromalacia patella is typically considered when there is a failure to improve with conservative treatment and rehab. The goal of surgery is to address the underlying structural or mechanical issues contributing to cartilage damage, and to utilise regenerative approaches to promote cartilage healing
Surgical approaches have moved away from isolated procedure (lateral retinaculum release, arthroscopic debridement or microfracture), towards combined techniques that address structural/ alignment issues with novel cartilage therapies.
Table – What’s hot in cartilage therapies of the knee
| Procedure name | Consider this for | Pro/cons |
| Allograft Matrices |
|
Pros
Biological Repair: Promotes natural cartilage regeneration, potentially restoring joint function and delaying arthritis progression. Suitable for Active Individuals: Helps young and active patients regain mobility and resume high-impact activities. ConsProlonged Recovery: Requires months of rehabilitation, with activity restrictions during healing. |
| Autologous Chondrocyte Implantation (ACI) |
|
Pros
Restores Cartilage Function: Regenerates hyaline-like cartilage, offering better durability and function compared to fibrocartilage formed by microfracture. Autologous: Native hyaline cartilage or natural bone-articular interface (Aspetar vid) Cons Limited Evidence for Patella: Success rates for patellofemoral cartilage defects are less well-established compared to femoral condyle defects. |
| Matrix-Induced Autologous Chondrocyte Implantation (MACI) |
|
Pros
Minimally Invasive Implantation: The bioresorbable matrix ensures stable fixation and easier graft handling during surgery Cons Selective Candidacy: Limited to patients under 50 years old with stable, well-aligned knees and no significant joint degeneration Excludes Degenerative Conditions: Ineffective for osteoarthritis or widespread cartilage loss |
| Bone Marrow Aspiration Chondrogenesis (BMAC) | Preference for Minimally Invasive Options: Patients seeking less invasive solutions or who are not candidates for more complex procedures like MACI
Defect Characteristics: Small to moderate cartilage defects compared to ACI/MACI Activity Level: Moderate activity demands, where long-term durability is less critical |
Pros
Faster Recovery: Compared to more invasive techniques like MACI or ACI Cons Cartilage Quality: Often produces fibrocartilage, which is less durable and mechanically inferior to hyaline cartilage Variable Outcomes: Results may be inconsistent and less durable over the long term compared to MACI |
| Osteochondral Allograft Transplantation (OCA) | Defect Characteristics: Large (>2 cm²), deep cartilage defects involving both cartilage and subchondral bone
Age: Young to middle-aged patients (under 50 years old) Previous Treatment: Suitable for patients who have failed other cartilage repair procedures (e.g., microfracture, ACI |
Pros
Single-Stage Procedure: Unlike staged techniques (e.g., ACI), OCA is completed in one surgery Cons Donor Graft Dependency: Requires availability of a matched cadaveric donor, which can delay surgery |
1. Allograft Matrices
Allograft matrices are bioengineered scaffolds derived from donor tissue that can provide a framework for cartilage regeneration in damaged areas. They are typically used in cases with significant cartilage loss where autologous options (from the patient’s own tissue) may not be suitable.
Procedure
- The matrix is implanted directly into the damaged area, either alone or with cellular components like chondrocytes or mesenchymal stem cells.
- The scaffold promotes cellular ingrowth and integration with the surrounding cartilage, helping the body form new cartilage tissue.
Outcomes
- Studies show that allograft matrices offer moderate to significant pain relief and improvements in joint functionality.
- Allograft matrices encourage the formation of hyaline-like cartilage, which is closer to the native cartilage structure compared to fibrocartilage.
- A study by Desai B et al (2024) found that significant improvements in different domains such as pain, functionality in sports and leisure as well as activities in daily living over 2 years were seen in those who underwent a single stage allograft matrix implantation.33
Allograft matrices provide a reliable option for cartilage repair, especially in larger defects or cases where autologous grafts are limited. They tend to deliver sustained improvement in pain and function for several years.
-
Autologous Chondrocyte Implantation (ACI)
A small sample of autologous articular cartilage is harvested from a non-weight bearing portion of the knee and sent to a lab. Chondrocytes are then collected through an enzymatic process and returned to the surgeon for implantation into affected area.
Outcomes
Pain Relief
- ACI has shown significant pain relief in patients with large or deep cartilage defects, especially in younger patients.
Functional Improvement:
- Studies have found that ACI can show early improvements in knee function with better range of motion during activities of daily living.
- A systematic review by Lauren et al (2023) found that patients were able to walk longer distances with significant improvement in strength up to 2 years post-operatively.34
- To preserve the strength benefits from ACI, long-term strength training may benefit patients.
Cartilage Regeneration:
- ACI promotes the regeneration of hyaline-like cartilage, which is biomechanically superior to fibrocartilage. This makes ACI one of the most promising cartilage repair techniques in terms of durability and long-term outcomes.
Long-Term Outcomes:
- ACI is associated with excellent long-term outcomes, particularly in terms of maintaining pain relief and joint function. Studies show that many patients continue to experience significant benefits 10-15 years post-surgery, with a lower likelihood of needing additional surgery compared to other procedures.
- Minas T et al (2014) found that ACI provided durable outcomes with improved function in 75% of patients with symptomatic cartilage defects at a minimum in 10 years post-surgery.35
ACI is a highly effective surgical treatment for chondropathy such as chondromalacia patella, particularly in young, active patients with large cartilage lesions. It provides long-term pain relief, functional improvement, and hyaline-like cartilage regeneration, making it one of the most durable options for cartilage repair. However, it is a technically demanding and costly procedure, typically reserved for cases where other treatments have failed.
Referral Criteria:
- Ideal Candidates: Patients with well-defined cartilage lesions >2 cm² in size, who have a stable, well-aligned knee with intact meniscus.
- Imaging: MRI is preferred for pre-procedure assessment.
- Exclusions: Patients with prior microfracture, malalignment, or instability (these issues must be corrected prior to ACI).
- Procedure Requirements: ACI is costly and usually funded only at specialised centres, necessitating a tertiary referral.36
Post-Operative Recovery:
- Weight Bearing:
- 2 weeks non-weight bearing (NWB)
- 4 weeks partial weight bearing (PWB)
- Full weight bearing (FWB) thereafter
- Range of Motion (ROM): Full ROM unless involving patellofemoral joint (PFJ).
- Bracing:
- 2 weeks in a cricket splint
- 4 weeks in an offloader brace
- Physical Therapy: To be customised for each patient.
- Antibiotics: Single pre-op dose.
- VTE Prophylaxis: 2 weeks of Dalteparin while NWB.
-
Matrix-Induced Autologous Chondrocyte Implantation (MACI)
Overview:
Matrix-Induced Autologous Chondrocyte Implantation (MACI) is an advanced cartilage repair technique that uses a bioresorbable matrix to support cultured autologous chondrocytes and delivers them directly into the cartilage defect. MACI is an improved version of traditional Autologous Chondrocyte Implantation (ACI), offering greater control over cell distribution and better mechanical stability.
Procedure:
- Harvesting: A small sample of cartilage is taken from a non-weight-bearing area of the patient’s knee.
- Cell Culturing: Chondrocytes from the sample are cultured in a lab to increase their numbers.
- Matrix Application: The cultured chondrocytes are embedded into a biodegradable collagen scaffold (matrix).
- Implantation: The matrix, now containing the chondrocytes, is shaped to fit the defect and implanted. It encourages even distribution of cells, stable fixation, and integration with surrounding tissue.
Outcomes
Pain Relief:
- Significant pain reduction, especially in patients with large, isolated cartilage defects. The structured scaffold supports cell growth, leading to better outcomes in pain relief compared to earlier ACI techniques.
- In the Ebert JR et al (2024) study of 82 patients who underwent MACI, 90.2% were satisfied with MACI in relieving their knee pain, 85.4% satisfied with their ability to participate in sports and 90.2% satisfied with the overall result of the surgery.37
Functional Improvement:
- MACI enables substantial improvements in knee function, particularly in young, active patients who are often able to return to their pre-injury activity levels. The matrix design provides greater stability and easier implantation, allowing for smoother integration with surrounding tissue.
Long-Term Outcomes:
- Ebert JR et al. (2024) study showed significant improvement in the range of active knee extension 10 years post-surgery.37
Referral Criteria
According to the NICE guidelines, the following criteria are often applied for MACI candidacy:
- Defect Size: Best suited for focal cartilage defects larger than 2 cm².
- Age: Typically younger, active patients (under 50 years) without degenerative joint changes.
- Knee Condition: Candidates should have a stable, well-aligned knee with an intact meniscus for optimal graft survival.
- Failed Conservative Treatment: Ideal for patients who have not responded to other non-surgical or minimally invasive treatments.
Exclusions:
- Degenerative Joint Disease: Limited success in patients with significant osteoarthritis.
- Uncorrected Malalignment or Instability: These issues should be addressed before considering MACI.
Post-Operative Recovery:
- Weight Bearing:
- Initial 2 weeks: Non-weight-bearing
- Subsequent 4 weeks: Partial weight-bearing
- Full weight-bearing thereafter
- Bracing:
- 2 weeks in a splint
- Followed by an offloader brace for an additional 4 weeks
- Physical Therapy: Individualized rehabilitation focused on gradually restoring strength and mobility.
MACI is a powerful, long-lasting solution for large cartilage defects, providing durable pain relief and improved knee function, though it is limited to patients who meet specific criteria. The procedure requires specialized centres and is best used after other treatments have been exhausted.
-
Bone Marrow Aspiration Chondrogenesis (BMAC)
Bone marrow aspiration concentrate (BMAC) chondrogenesis involves extracting bone marrow from the patient, concentrating it to isolate mesenchymal stem cells (MSCs), and then injecting these cells into the cartilage defect. MSCs are multipotent, meaning they can differentiate into various cell types, including chondrocytes, which form cartilage.
Procedure
- Bone marrow is aspirated (often from the iliac crest), processed to isolate MSCs, and then concentrated.
- The concentrated MSCs are implanted into the cartilage defect, either with a scaffold or injected alone.
- MSCs in the area can differentiate into chondrocytes and contribute to cartilage regeneration.
Outcomes
- Pain Relief: BMAC has shown positive results in reducing pain, particularly in patients with small to moderate cartilage defects. Pain relief tends to be less pronounced than in ACI or MACI.
- Functional Improvement: Improvement in function has been observed, especially in younger patients with isolated defects. However, results may vary depending on defect size and joint environment.
- Cartilage Regeneration: BMAC stimulates some degree of cartilage regeneration, though it may not yield hyaline-like cartilage comparable to ACI or MACI. The cartilage formed is often fibrocartilage, which is less durable but still offers symptomatic relief.
BMAC is a promising, minimally invasive option for patients with smaller cartilage defects or when other surgical options are not feasible. However, BMAC tends to produce fibrocartilage rather than true hyaline cartilage, limiting its long-term durability in high-demand patients.38
-
Osteochondral Allograft Transplantation (OCA)
Overview:
OCA involves harvesting non-weight bearing portions of articular cartilage and their underlying bone and implanting this into the symptomatic and defective lesion of the same knee. This procedure is particularly useful for treating large, deep cartilage lesions that extend into the bone. OCA provides both a cartilage surface and structural bone support in a single procedure, making it effective for complex defects.
According to NICE guidelines, OCA may be recommended for specific cases of cartilage damage in the knee:
- Lesion Size: Defects larger than 2 cm², where the lesion involves both cartilage and subchondral bone.
- Previous Treatment Failure: Ideal for patients who have not responded to other cartilage repair procedures, such as microfracture or autologous chondrocyte implantation (ACI).
- Age: Typically considered for young to middle-aged patients (under 50 years old) who are otherwise in good health, as this group tends to have better long-term outcomes with OCA.
- Knee Condition: The knee should be well-aligned, stable, and have an intact meniscus to maximize graft survival and functionality.
- Absence of Degenerative Changes: Patients should have minimal to no osteoarthritis in the joint, as degenerative conditions may decrease the success of the graft.
Exclusions:
- Severe Osteoarthritis: Patients with advanced osteoarthritis or generalized joint degeneration are typically not candidates for OCA.
- Inadequate Donor Graft Availability: The procedure requires a matched donor graft, which can sometimes delay treatment.
Procedure:
- Graft Harvest and Preparation: An osteochondral allograft containing cartilage and subchondral bone is harvested from a cadaver donor and prepared for the patient’s defect.
- Implantation: The graft is placed into the knee and secured, where it can integrate with the native cartilage and bone.
Outcomes and Effectiveness
- Pain Relief: Studies report significant, long-lasting pain relief.
- Functional Improvement: OCA enables improved knee function, particularly in active patients.
- Cartilage & Bone Integration: High rates of graft integration and stable incorporation have been observed.
- Long-Term Durability: Many patients maintain positive outcomes for 5-10 years
OCA is a highly effective treatment for large, complex cartilage defects with bone involvement, offering durable pain relief and functional recovery. It is ideal for young, active patients with well-defined, stable lesions and minimal degenerative changes. However, it may be limited by donor availability and requires careful patient selection to ensure optimal outcomes.39
Conclusions
- Chondromalacia patella is a radiological diagnosis, usually seen on MRI – due to abnormal or excessive loading on the cartilage surface of the knee cap.
- Chondromalacia patella rarely occurs by itself, and there may be several co-existing pain drivers.
- MSK clinics shoulder consider mechanical triggers (trauma/ overuse), alignment (hypermobility, and tracking) and the metabolic potential for the cartilage to heal as part of the work up.
- Conservative treatments options should focus on optimising joint forces, alignment, rehab, and injection therapies for pain relief.
- New surgical cartilage therapies are available to help support the health of the knee alongside procedure to optimise alignment.
Authors and Affiliations
Dr Tamer Ahamed, Dr Salmaan Ahmed, Muhammed Umer, Dr Ryan Linn, Dr Jeffrey Peng, Dr Oran Roche, Dr Irfan Ahmed, Mr Arman Memarzadeh
Dr Tamer Ahamed
FY2 Doctor
Basildon & Thurrock University Hospital
LinkedIn: www.linkedin.com/in/tamer-ahamed-ab1779178/
Dr Salmaan Ahmed
FY1 Doctor
Whipps Cross University Hospital
LinkedIn: www.linkedin.com/in/salmaan-ahmed-190644a7
Muhammed Umer
5th Year Medical Student
King’s College London
LinkedIn: https://uk.linkedin.com/in/muhammad-umer19
Dr Ryan Linn
FY1 Doctor
University College London
Twitter: @Ryan_Linn_
Dr Jeffrey Peng MD, CAQSM
Sports Medicine Physician
Clinical Assistant Professor (Affiliated); Stanford University School of Medicine, Department of Medicine, Division of Primary Care & Population Health
Twitter: @JeffreyPengMD
YouTube: youtube.com/c/JeffreyPengMD
Website: www.JeffreyPengMD.com
Dr Oran Roche
Consultant MSK Radiologist
Luton & Dunstable University Hospital
Dr Irfan Ahmed
Consultant in Musculoskeletal, Sport & Exercise Medicine (SEM)
MBBS, MA (Cantab), MSc, FFSEM, PG cert MSK Ultrasound
Www.mskplaybook.com
Twitter: @ExerciseIrfan
Mr Arman Memarzadeh
Consultant in Trauma and Orthopaedics, Knee Surgery Specialist
MBBS, FRCS (Tr and Orth), PGCME
Cambridge University Hospitals
Twitter: @MyKneeSurgeon
YouTube: https://www.youtube.com/@MyKneeSurgeon
LinkedIn: www.linkedin.com/in/mykneesurgeon
Website: https://mykneesurgeon.com
References
- Habusta SF, Coffey R, Ponnarasu S, Mabrouk A, Griffin EE. Chondromalacia Patella. StatPearls [Internet]. 2023 Apr 22 [cited 2024 Nov 23]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK459195/
- Zheng W, Li H, Hu K, Li L, Bei M. Chondromalacia patellae: current options and emerging cell therapies. Stem Cell Res Ther [Internet]. 2021 Dec 1 [cited 2024 Nov 23];12(1):412. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8287755/
- Zhang H, Kong X qing, Cheng C, Liang M hua. A correlative study between prevalence of chondromalacia patellae and sports injury in 4068 students. Chin J Traumatol. 2003 Dec;6(6):370–4.
- Leslie IJ, Bentley G. Arthroscopy in the diagnosis of chondromalacia patellae. Ann Rheum Dis [Internet]. 1978 Dec 1 [cited 2024 Nov 24];37(6):540–7. Available from: https://ard.bmj.com/content/37/6/540
- Heintjes EM, Berger M, Bierma-Zeinstra SMA, Bernsen RMD, Verhaar JAN, Koes BW. Pharmacotherapy for patellofemoral pain syndrome. Cochrane Database Syst Rev [Internet]. 2004 Jul 19 [cited 2024 Nov 23];2004(3):CD003470. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8276350/
- Sharma R, Vaibhav V, Meshram R, Singh B, Khorwal G. A Systematic Review on Quadriceps Angle in Relation to Knee Abnormalities. Cureus [Internet]. 2023 Jan 30 [cited 2024 Nov 24];15(1):e34355. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9974941/
- Smith BE, Selfe J, Thacker D, Hendrick P, Bateman M, Moffatt F, et al. Incidence and prevalence of patellofemoral pain: A systematic review and meta-analysis. PLoS One [Internet]. 2018 Jan 1 [cited 2024 Nov 24];13(1):e0190892. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC5764329/
- Cai Y, Deng Y, Ou L, Guo Y, Guo Y. Clinical trial of manual therapy in the treatment of chondromalacia patellae. Medicine [Internet]. 2023 Jun 16 [cited 2024 Nov 24];102(24):e33945. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10270499/
- Hart HF, Patterson BE, Crossley KM, Culvenor AG, Khan MCM, King MG, et al. May the force be with you: understanding how patellofemoral joint reaction force compares across different activities and physical interventions—a systematic review and meta-analysis. Br J Sports Med [Internet]. 2022 May 1 [cited 2024 Nov 23];56(9):521–30. Available from: https://bjsm.bmj.com/content/56/9/521
- Knee Biomechanics – Recon – Orthobullets [Internet]. [cited 2024 Nov 23]. Available from: https://www.orthobullets.com/recon/9065/knee-biomechanics
- Gagliardi JA, Chung EM, Chandnani VP, Kesling KL, Christensen KP, Null RN, et al. Detection and staging of chondromalacia patellae: relative efficacies of conventional MR imaging, MR arthrography, and CT arthrography. AJR Am J Roentgenol [Internet]. 1994 [cited 2024 Nov 24];163(3):629–36. Available from: https://pubmed.ncbi.nlm.nih.gov/8079858/
- Kim H joo, Lee SH, Kang CH, Ryu JA, Shin MJ, Cho KJ, et al. Evaluation of the chondromalacia patella using a microscopy coil: comparison of the two-dimensional fast spin echo techniques and the three-dimensional fast field echo techniques. Korean J Radiol [Internet]. 2011 Jan [cited 2024 Nov 24];12(1):78–88. Available from: https://pubmed.ncbi.nlm.nih.gov/21228943/
- Lee SH, Suh JS, Cho J, Kim SJ, Kim SJ. Evaluation of chondromalacia of the patella with axial inversion recovery-fast spin-echo imaging. J Magn Reson Imaging [Internet]. 2001 [cited 2024 Nov 24];13(3):412–6. Available from: https://pubmed.ncbi.nlm.nih.gov/11241815/
- Macarini L, Perrone A, Murrone M, Marini S, Stefanelli M. Evaluation of patellar chondromalacia with MR: comparison between T2-weighted FSE SPIR and GE MTC. Radiol Med. 2004 Sep;108(3):159–71.
- van Eck CF, Kingston RS, Crues J V., Kharrazi FD. Magnetic Resonance Imaging for Patellofemoral Chondromalacia: Is There a Role for T2 Mapping? Orthop J Sports Med [Internet]. 2017 Nov 16 [cited 2024 Nov 24];5(11). Available from: https://pubmed.ncbi.nlm.nih.gov/29204454/
- Vanarthos WJ, Pope TL, Monu JU. Comparison of axial T1 spin-echo and T1 fat-saturation magnetic resonance imaging techniques in the diagnosis of chondromalacia patellae. Orthop Rev. 1994 Dec;23(12):942–6.
- Quatman CE, Hettrich CM, Schmitt LC, Spindler KP. The Clinical Utility and Diagnostic Performance of MRI for Identification of Early and Advanced Knee Osteoarthritis: A Systematic Review. Am J Sports Med [Internet]. 2011 Jul [cited 2024 Nov 24];39(7):1557. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3782308/
- Mattila VM, Weckström M, Leppänen V, Kiuru M, Pihlajamäki H. Sensitivity of MRI for articular cartilage lesions of the patellae. Scand J Surg [Internet]. 2012 Mar 1 [cited 2024 Nov 24];101(1):56–61. Available from: https://pubmed.ncbi.nlm.nih.gov/22414470/
- Cho J, Yi J, Song Y, Kim YU. Assessment of patellar cartilage cross-sectional area in patients with lower grade chondromalacia patella. Medicine [Internet]. 2023 Aug 18 [cited 2024 Nov 24];102(33):e34307. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10443766/
- Kızılgöz V, Kantarci M, Aydın S. Association between the subcutaneous fat thickness of the knee and chondromalacia patella: a magnetic resonance imaging-based study. J Int Med Res [Internet]. 2023 Jun 1 [cited 2024 Nov 24];51(6):03000605231183581. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC10328038/
- Khoo P, Ghoshal A, Byrne D, Subramaniam R, Moran R. A novel clinical test for assessing patellar cartilage changes and its correlation with magnetic resonance imaging and arthroscopy. Physiother Theory Pract [Internet]. 2019 Aug 3 [cited 2024 Nov 24];35(8):781–6. Available from: https://pubmed.ncbi.nlm.nih.gov/29601214/
- Doberstein ST, Romeyn RL, Reineke DM. The diagnostic value of the Clarke sign in assessing chondromalacia patella. J Athl Train [Internet]. 2008 [cited 2024 Nov 24];43(2):190–6. Available from: https://pubmed.ncbi.nlm.nih.gov/18345345/
- Chamorro-Moriana G, Espuny-Ruiz F, Ridao-Fernández C, Magni E. Clinical value of questionnaires & physical tests for patellofemoral pain: Validity, reliability and predictive capacity. PLoS One [Internet]. 2024 Apr 1 [cited 2024 Nov 24];19(4). Available from: https://pubmed.ncbi.nlm.nih.gov/38630735/
- Sisk D, Fredericson M. Taping, Bracing, and Injection Treatment for Patellofemoral Pain and Patellar Tendinopathy. Curr Rev Musculoskelet Med [Internet]. 2020 Aug 1 [cited 2024 Nov 25];13(4):537. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7340692/
- Maia PAV, Cossich VRA, Salles-Neto JI, Aguiar DP, de Sousa EB. Viscosupplementation improves pain, function and muscle strength, but not proprioception, in patients with knee osteoarthritis: a prospective randomized trial. Clinics (Sao Paulo) [Internet]. 2019 [cited 2024 Nov 25];74. Available from: https://pubmed.ncbi.nlm.nih.gov/31778431/
- Zhang S, Jia M, Luo Y, Wang X, Shi Z, Xiao J. [Hyaluronate acid for treatment of chondromalacia patellae: a 52-week follow-up study]. Nan Fang Yi Ke Da Xue Xue Bao [Internet]. 2019 Jul 20 [cited 2024 Nov 25];39(7):791–6. Available from: https://pubmed.ncbi.nlm.nih.gov/31340911/
- Park JG, Sim J, Han SB. Association between intra-articular hyaluronic acid injections in delaying total knee arthroplasty and safety evaluation in primary knee osteoarthritis: analysis based on Health Insurance Review and Assessment Service (HIRA) claim database in Republic of Korea. BMC Musculoskelet Disord [Internet]. 2024 Dec 1 [cited 2025 Jan 1];25(1):1–11. Available from: https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-024-07698-2
- Filardo G, Previtali D, Napoli F, Candrian C, Zaffagnini S, Grassi A. PRP Injections for the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomized Controlled Trials. Cartilage [Internet]. 2021 Dec 1 [cited 2025 Jan 1];13(1_suppl):364S-375S. Available from: https://pubmed.ncbi.nlm.nih.gov/32551947/
- Qiao X, Yan L, Feng Y, Li X, Zhang K, Lv Z, et al. Efficacy and safety of corticosteroids, hyaluronic acid, and PRP and combination therapy for knee osteoarthritis: a systematic review and network meta-analysis. BMC Musculoskelet Disord [Internet]. 2023 Dec 1 [cited 2025 Jan 1];24(1). Available from: https://pubmed.ncbi.nlm.nih.gov/38037038/
- Li M, Zhang C, Ai Z, Yuan T, Feng Y, Jia W. [Therapeutic effectiveness of intra-knee-articular injection of platelet-rich plasma on knee articular cartilage degeneration]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 Oct;25(10):1192–6.
- Ostojic M, Hakam HT, Lovrekovic B, Ramadanov N, Prill R. Treatment of anterior knee pain due to chondromalacia patellae with platelet-rich plasma and hyaluronic acid in young and middle-aged adults, a cohort study. Arch Orthop Trauma Surg [Internet]. 2024 [cited 2024 Nov 25];144(9). Available from: https://pubmed.ncbi.nlm.nih.gov/38780774/
- Pak J, Lee JH, Lee SH. A novel biological approach to treat chondromalacia patellae. PLoS One [Internet]. 2013 May 20 [cited 2024 Nov 25];8(5). Available from: https://pubmed.ncbi.nlm.nih.gov/23700485/
- Desai B, Assid E, Jacobs G, Dasgupta A, Williams G, Choate WS, et al. Viable cartilage allograft outperforms existing treatments for focal knee cartilage defects. Knee Surg Sports Traumatol Arthrosc [Internet]. 2024 Mar 1 [cited 2024 Nov 25];32(3):636–44. Available from: https://pubmed.ncbi.nlm.nih.gov/38391111/
- Smith L, Jakubiec A, Biant L, Tawy G. The biomechanical and functional outcomes of autologous chondrocyte implantation for articular cartilage defects of the knee: A systematic review. Knee [Internet]. 2023 Oct 1 [cited 2024 Nov 25];44:31–42. Available from: https://pubmed.ncbi.nlm.nih.gov/37516029/
- Minas T, Von Keudell A, Bryant T, Gomoll AH. The John Insall Award: A minimum 10-year outcome study of autologous chondrocyte implantation. Clin Orthop Relat Res [Internet]. 2014 [cited 2024 Nov 25];472(1):41–51. Available from: https://pubmed.ncbi.nlm.nih.gov/23979923/
- 3 Committee discussion | Autologous chondrocyte implantation for treating symptomatic articular cartilage defects of the knee | Guidance | NICE [Internet]. [cited 2024 Nov 25]. Available from: https://www.nice.org.uk/guidance/ta477/chapter/3-Committee-discussion
- Ebert JR, Klinken S, Fallon M, Wood DJ, Janes GC. Clinical and Radiological Outcomes at ≥10-Year Follow-up After Matrix-induced Autologous Chondrocyte Implantation in the Patellofemoral Joint. Am J Sports Med [Internet]. 2024 Aug 1 [cited 2024 Nov 25];52(10). Available from: https://pubmed.ncbi.nlm.nih.gov/39101611/
- Madry H, Gao L, Eichler H, Orth P, Cucchiarini M. Bone Marrow Aspirate Concentrate-Enhanced Marrow Stimulation of Chondral Defects. Stem Cells Int [Internet]. 2017 Jan 1 [cited 2024 Nov 25];2017(1):1609685. Available from: https://onlinelibrary.wiley.com/doi/full/10.1155/2017/1609685
- Chahla J, Sweet MC, Okoroha KR, Nwachukwu BU, Hinckel B, Farr J, et al. Osteochondral Allograft Transplantation in the Patellofemoral Joint: A Systematic Review. https://doi.org/101177/0363546518814236 [Internet]. 2018 Dec 7 [cited 2024 Nov 25];47(12):3009–18. Available from: https://journals.sagepub.com/doi/10.1177/0363546518814236