A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: is trimethoprim-sulfamethoxazole a real option?
Abstract
Objectives
The objective of this study was to perform a systematic review and meta-analysis of the literature to evaluate the efficacy and safety of therapies for cerebral toxoplasmosis in HIV-infected adults. The pyrimethamine plus sulfadiazine (P-S) combination is considered the mainstay therapy for cerebral toxoplasmosis and pyrimethamine plus clindamycin (P-C) is the most common alternative treatment. Although trimethoprim-sulfamethoxazole (TMP-SMX) has potential advantages, its use is infrequent.
Methods
We searched PubMed and four other databases to identify randomized controlled trials (RCTs) and cohort studies. Two independent reviewers searched the databases, identified studies and extracted data. Risk ratios (RRs) were pooled across studies using random-effects models.
Results
Nine studies were included (five RCTs, three retrospective cohort studies and one prospective cohort study). In comparison to P-S, treatment with P-C or TMP-SMX was associated with similar rates of partial or complete clinical response [P-C: RR 0.87; 95% confidence interval (CI) 0.70–1.08; TMP-SMX: RR 0.97; 95% CI 0.78–1.21], radiological response (P-C: RR 0.92; 95% CI 0.82–1.03), skin rash (P-C: RR 0.81; 95% CI 0.56–1.17; TMP-SMX: RR 0.17; 95% CI 0.02–1.29), gastrointestinal impairment (P-C: RR 5.16; 95% CI 0.66–40.11), and drug discontinuation because of adverse events (P-C: RR 0.32; 95% CI 0.07–1.47). Liver impairment was more frequent with P-S than P-C (P-C vs. P-S: RR 0.48; 95% CI 0.24–0.97).
Conclusions
The current evidence fails to identify a superior regimen in terms of relative efficacy or safety for the treatment of HIV-associated cerebral toxoplasmosis. Use of TMP-SMX as preferred treatment may be consistent with the available evidence and other real-world considerations. Larger comparative studies are needed.
Introduction
Highly active antiretroviral therapy (HAART) has significantly reduced the incidence, prevalence, and case-fatality rates of cerebral toxoplasmosis in HIV-infected patients from both developed and developing countries 1, 2. Treatment of cerebral toxoplasmosis has been relatively successful in comparison to other opportunistic infections; however, cerebral toxoplasmosis still represents a poor prognostic determinant in patients with advanced disease and severe immunodeficiency 1, 3, 4.
Although the first case of HIV-associated cerebral toxoplasmosis was described in 1980 5, there have been only a few randomized controlled trials and cohort studies comparing different regimens for the treatment of AIDS-associated cerebral toxoplasmosis 6, 7. Thus, although HIV-associated cerebral toxoplasmosis is quite a common world-wide problem, there is a scarcity of evidence regarding optimal therapy. The preferred initial therapy for cerebral toxoplasmosis in Department of Health and Human Services of the USA (DHHS), European AIDS Clinical Society (EACS), and British HIV Association (BHIVA) guidelines is the combination of pyrimethamine plus sulfadiazine (P-S), and pyrimethamine plus clindamycin (P-C) is the preferred alternative regimen 8-10. Trimethoprim-sulfamethoxazole (TMP-SMX) usually appears as an additional alternative in these three guidelines, but other recommendations 11 indicate this combination and P-S as first-line therapies. In clinical practice, however, TMP-SMX use is infrequent when P-C and P-S are available. In contrast, TMP-SMX is the first choice in Africa and in other developing regions, particularly where other regimens are not available or where there is experience with TMP-SMX. TMP-SMX has potential advantages (i.e. tolerability, posology, parenteral formulation and accessibility) and remains an attractive option. Pyrimethamine is a standard use drug approved by the Food and Drug Administration (FDA) in 1953 but its price has increased worryingly, with a negative impact on both public health and individual patient care. In addition, regimens including pyrimethamine require the use of folinic acid, a medication usually unavailable in most resource-limited settings. The DHHS guidelines mention that pyrimethamine will soon no longer be available in retail pharmacies in the USA (http://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/0) and recommends it for patients with suspected or documented toxoplasmosis who do not have a history of sulfa allergy; TMP-SMX should be used as a substitute if pyrimethamine is not readily available. In this scenario, the optimal management of cerebral toxoplasmosis continues to be controversial, and evidence-based, clear alternatives to pyrimetamine are needed.
The purpose of this systematic review and meta-analysis was to evaluate the most effective and safest therapy for HIV-associated cerebral toxoplasmosis.
Methods
Data sources and searches
We performed a systematic search for publications using PubMed-Medline, CINAHL, Scopus, Web of Science and the Cochrane Library from database inception to 30 October 2014. Search strategies used subject headings and keywords with no language restrictions. The database searches were performed independently by two authors (PT and DP). The PubMed search strategy is available as supplementary data (Appendix S1). A manual search of references from recent reviews and relevant published original studies was also performed to identify relevant studies.
The following predetermined inclusion criteria were used: (1) studies evaluating the efficacy and safety of acute therapeutic regimens in HIV-infected patients with cerebral toxoplasmosis; (2) study population of patients > 18 years old. Our exclusion criteria were: (1) single drug regimen observational studies; (2) case series; (3) studies in which safety and efficacy data were not available or could not be extracted for each of the study groups. Efficacy outcomes of interest were clinical and radiological response. All adverse event data provided in the included studies were recorded for safety outcomes.
Study selection and data extraction
A list of retrieved articles was reviewed independently by two investigators (PT and DP) to choose potentially relevant articles, and any disagreements were discussed and resolved by consensus with one other author (AVH). Two reviewers (PT and DP) independently extracted and recorded data from included studies. The following information was extracted: age, gender, time since AIDS diagnosis, treatment regimens, duration of treatment, follow-up time, clinical and radiological responses, and adverse events. One other author (AVH) reviewed the extractions for discrepancies, and the three authors (PT, DP and AVH) reached a consensus.
Evaluation of study quality
The quality of all included cohort studies was assessed independently by two authors (PT and VP) using the Newcastle–Ottawa scale (NOS) 12. The NOS uses two different tools for case–control and cohort studies and consists of three parameters of quality: selection, comparability and exposure/outcome assessment. The NOS assigns a maximum of four points for selection, two points for comparability and three points for exposure or outcome. NOS scores of ≥ 7 were considered to represent high-quality studies and NOS scores of 5–6 were considered to represent moderate quality.
The quality of all included randomized controlled trials (RCTs) was assessed independently by two authors (PT and VP) using The Cochrane Collaboration tool for assessing risk of bias 13. The following items were evaluated: generation of the allocation sequence (selection bias); concealment of the allocation sequence (selection bias); blinding (detection and performance bias); blinding of participants and personnel to outcome assessment; incomplete outcome data (attrition bias); selective outcome reporting (reporting bias); and other biases. For each RCT, each item was described as having either a low risk of bias, a high risk of bias, or an unclear risk of bias. All discrepancies were addressed by a re-evaluation of the original article as a group (PT, VP and AVH).
Data synthesis and analysis
Our systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) collaboration 14. DerSimonian and Laird random-effects models and the inverse variance method were used for all meta-analyses 15 to calculate pooled risk ratios and 95% confidence intervals (CIs). I2 values of 30–60% represented a moderate level of heterogeneity. A P-value of < 0.1 for χ2 was defined as indicating the presence of heterogeneity. Publication bias was illustrated using funnel plots and formally tested with Egger's test 16. We used Review Manager for all statistical analyses (RevMan 5.0; The Cochrane Collaboration, Oxford, UK; http://tech.cochrane.org/revman/about-revman-5).
Results
Eligible studies
Of the 504 unique articles retrieved and screened by study title, 30 potentially relevant articles were selected based on relevance to the study topic. After screening the abstracts, 16 articles were found to fulfil the inclusion criteria and were selected for full-text review (Fig. 1). Nine articles (n = 692) assessing the efficacy and/or safety of acute therapeutic regimens in HIV-infected patients with cerebral toxoplasmosis were included in our systematic review and meta-analysis. Seven articles were excluded after full-text review (reasons for exclusion are listed in Fig. 1).
Study characteristics
Table 1 summarizes the main characteristics of the included studies. Of the nine included studies, five were RCTs 17-21, three were retrospective cohort studies 1, 22, 23 and one was a prospective cohort study 24. Sample size ranged from 29 to 299 patients. The most commonly evaluated treatment regimen was P-S, which was one of the treatment arms in seven studies. Duration of treatment varied from 3 weeks to 6–8 weeks.
| Authors, year published | Study location | Type of study | Sample size | Age [mean (SD)] | Male (%) | Treatment groups; dosage | Duration of treatment | |
|---|---|---|---|---|---|---|---|---|
| Antinori et al., 1992 1 | Italy | RC | 29 | 34.4 (7.4) | 74.4 |
Pyrimethamine plus sulfadiazine; 50–75 mg/day, 4–6 g/day |
Pyrimethamine i.v. plus clindamycin; 50–75 mg/day, 2.4–3.6 g/day |
6–8 weeks |
| Arens et al., 2007 21 | South Africa | RC | 43 | 32.6 | 41.9 |
Pyrimethamine plus sulfadiazine; NR |
Trimethoprim plus sulfamethoxazole; NR |
NR |
| Caumes et al., 1995 22 | France | RC | 55 | 38.0 | 81.8 | Pyrimethamine plus sulfadiazine; 100 mg loading dose, then 50 mg/day, 4 g/day | Pyrimethamine plus clindamycin; 100 mg loading dose, then 50 mg/day, 2.4 g/day | 6–8 weeks |
| Chirgwin et al.,2002 16 | France, USA | RCT | 49 | 35.6 | 65.3 | Atovaquone plus pyrimethamine; 3 g/day, 200 mg loading dose then 75 mg/day | Atovaquone plus sulfadiazine; 3 g/day, 4–6 g/day | 6 weeks |
| Dannemann et al., 1992 17 | USA, Europe | RCT | 59 | 36.0 (8.5) | 84.8 | Pyrimethamine plus sulfadiazine; 200 mg loading dose, then 75 mg/day, 100 mg/kg up to 8 g/day | Pyrimethamine plus clindamycin; 200 mg loading dose, then 75 mg/day, 4.8 g/day i.v. 3 weeks and then 1.2–1.35 g/day orally 3 weeks | 6 weeks |
| Katlama et al., 1996 18 | Europe | RCT | 299 | 33.5 (8.5) | 87.6 | Pyrimethamine plus sulfadiazine; 50 mg/day, 4 g/day | Pyrimethamine plus clindamycin; 50 mg/day,2.4 g/day | 6 weeks |
| Kongsaengdao et al., 2008 20 | Thailand | RCT | 30 | 37.3 (9.8) | 63.3 | Pyrimethamine plus sulfadiazine; 50–100 mg/day, 4 g/day | Trimethoprim plus sulfamethoxazole; 10 mg/kg/day, 50 mg/kg/day | 6 weeks |
| Ruf & Pohle, 1991 23 | Germany | PC | 51 | NR | NR | Pyrimethamine plus clindamycin plus spiramycin; 1.5 mg/kg/day, 2.4 g/day; 9x106 IU | Pyrimethamine plus clindamycin; 50–75 mg/day, 2.4 g/day | 3 weeks |
| Torre et al., 1998 20 | Italy | RCT | 77 | 33.2 (5.6) | 74.0 | Pyrimethamine plus sulfadiazine; 50 mg/day, 60 mg/kg/day | Trimethoprim plus sulfamethoxazole; 10 mg/kg/day, 50 mg/kg/day | 30 daysa |
- i.v., intravenous; IU, international units; RC, retrospective cohort; PC, prospective cohort; RCT, randomized controlled trial; NR, not reported; SD, standard deviation.
- a Followed by 50% maintenance dose for 3 months.
Quality assessment and publication bias
NOS quality scores for cohort studies ranged from 6 to 8 (Supporting Information Table S1a). The majority of the included RCTs were assessed as having a high risk of bias using the Cochrane tool, with no blinding of participants, personnel and outcome assessment as the main issues (Table S1b). The low number of studies in the pooled analyses (< 10) precluded us from evaluating for publication bias by funnel plot analysis. However, publication bias was minimized by a comprehensive search of databases without language and publication date restrictions.
Meta-analyses
Clinical and radiological efficacy
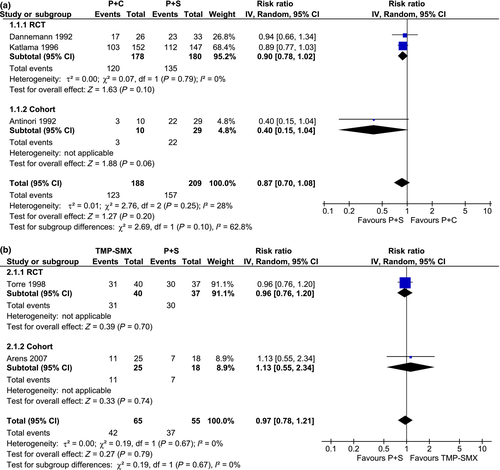
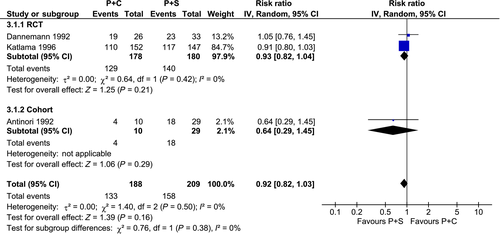
Treatment with P-S had a similar clinical efficacy when compared with P-C or TMP-SMX, with similar rates of partial or complete clinical response [P-C vs. P-S: risk ratio (RR) 0.87; 95% confidence interval (CI) 0.70–1.08; I2 = 28%; TMP-SMX vs. P-S: RR 0.97; 95% CI 0.78–1.21; I2 = 0%] (Fig. 2) and radiological response (P-C vs. P-S: RR 0.92; 95% CI 0.82–1.03; I2 = 0%) (Fig. 3).
Safety
The rates of skin rash (P-C vs. P-S: RR 0.81; 95% CI 0.56–1.17; I2 = 53%; TMP-SMX vs. P-S: RR 0.17; 95% CI 0.02–1.29; I2 = 0%) (Figure S1), gastrointestinal impairment (P-C vs. P-S: RR 5.16; 95% CI 0.66–40.11; I2 = 56%) (Figure S2), and drug discontinuation because of adverse events (P-C vs. P-S: RR 0.32; 95% CI 0.07–1.47; I2 = 42%) (Figure S3) were similar for P-S vs. P-C or TMP-SMX. Liver impairment was more frequent for P-S vs. P-C (P-C vs. P-S: RR 0.48; 95% CI 0.24–0.97) (Figure S4).
Discussion
In this systematic review and meta-analysis, we found that treatment with P-S had similar relative clinical and radiological efficacy and similar safety when compared with P-C or TMP-SMX. Among adverse events, only liver impairment was more frequent with P-S when compared with the P-C regimen.
The main objective of this study was to compare and evaluate the current evidence on various treatment regimens for cerebral toxoplasmosis and to explore the availability of alternative, better treatment regimens. Table 2 presents an overview of two previous reviews and the present study. The first study was a systematic review by The Cochrane Collaboration 6. This review included only randomized double-blind trials. On evaluating three studies, two comparing P-S with P-C 18, 19 and one comparing P-S with TMP-SMX 21, the authors found similar rates of complete or partial clinical response between the regimens. The second study was a systematic review and meta-analysis 7, which included randomized and quasi-randomized controlled studies. On analysing five studies, three comparing P-S with P-C 1, 18, 19 and two comparing P-S with TMP-SMX 20, 21, the authors found similar rates of complete or partial clinical response and mortality outcomes between the regimens. Interestingly, P-S showed substantial toxicity and intolerance when compared with P-C.
| Dedicoat et al. 6 | Yan et al. 7 | Our study | |
|---|---|---|---|
| Year of publication | 2006 | 2013 | – |
| Aim | To determine the most effective therapy for TE in HIV-infected adults | To determine the therapy that is most effective for primary prophylaxis of TE or for treating HIV-infected patients presenting with the first episode TE | To evaluate the most effective and safe therapy for cerebral toxoplasmosis in HIV-infected adults |
| Type of studies included | Randomized double-blinded trials | Randomized and quasi-randomized controlled trials | RCTs and cohort studies |
| Search databases | PubMed, EMBASE, AIDSearch and The Cochrane Library | PubMed, Google Scholar, EMBASE and CENTRAL | PubMed, CINAHL, Scopus, Web of Science and The Cochrane Library |
| Search cut-off date | November 2005 | September 2012 | October 2014 |
| Number of studies included | Three trials | 11 trials (including five studies evaluating treatment of TE) | Five RCTs, three retrospective cohort studies and one prospective cohort study |
| Total number of patients | 435 | 1472 (n = 494 in studies evaluating treatment of TE) | 692 |
| Study quality assessment | The assessment was based on the quality of allocation concealment, blinding, baseline characteristics of patients, use of intention-to-treat analysis and completeness of follow-up | Jadad scale | Cochrane risk of bias tool for RCTs; Newcastle−Ottawa scale for cohort studies |
| Primary outcome | Mortality, clinical response to treatment, neurological outcome and serious adverse events | TE (when prophylactics used), mortality and drug-limiting toxicity | Clinical and radiological efficacy of treatment and adverse effects |
| Results |
Mortality (P-C vs. P-S: RR 1.41; 95% CI 0.88–2.28; P-S vs. TMP-SMX: RR 0.0; 95% CI 0.0–0.0); partial or complete clinical response (P-C vs. P-S: RR 0.95; 95% CI 0.55–1.64; P-S vs. TMP-SMX: RR 1.00; 95% CI 0.74–1.33); adverse events (P-C vs. P-S: RR 0.95; 95% CI 0.81–1.11; P-S vs. TMP-SMX: RR 0.58; 95% CI 0.21–1.61); radiological response (P-C vs. P-S: RR 0.95; 95% CI 0.84–1.07; P-S vs. TMP-SMX: RR 1.09; 95% CI 0.78–1.51) |
Episodes of TE (TMP-SMX vs. D-P: OR 0.98; 95% CI 0.48–2.00); mortality (TMP-SMX vs. D-P: OR 0.75; 95% CI 0.53–1.06; P-S vs. P- C: OR 0.66; 95% CI 0.37–1.17; P-S vs. TMP-SMX: OR 0.12; 95% CI 0.01–1.39); toxicity and intolerance (TMP-SMX vs. D-P: OR 1.47; 95% CI 0.91–2.38; P-S vs. P-C: OR 3.08; 95% CI 1.82–5.24; P-S vs. TMP-SMX: OR 2.91; 95% CI 0.99–8.55); clinical response (P-S vs. P-C: OR 1.63; 95% CI 1.05–2.51; P-S vs. TMP-SMX: OR 0.90; 95% CI 0.39–2.06) |
Partial or complete clinical response (P-C vs. P-S: RR 0.87; 95% CI 0.70–1.08; TMP-SMX vs. P-S: RR 0.97; 95% CI 0.78–1.21); radiological response (P-C vs. P-S: RR 0.92; 95% CI 0.82–1.03); skin rash (P-C vs. P-S: RR 0.81; 95% CI 0.56–1.17; TMP-SMX vs. P-S: RR 0.17; 95% CI 0.02–1.29); GI impairment (P-C vs. P-S: RR 5.16; 95% CI 0.66–40.11; I2 = 56%); drug discontinuation because of adverse events (P-C vs. P–S: RR 0.32; 95% CI 0.07–1.47); liver impairment (P-C vs. P-S: RR 0.48; 95% CI 0.24–0.97) |
| Conclusions | The available evidence fails to identify any one superior regimen for the treatment of TE. TMP-SMX appears to be an effective alternative therapy for TE in resource-poor settings where pyrimethamine and sulfadiazine are not available. | The available evidence fails to identify any one superior regimen for the primary prophylaxis and treatment of TE. Administration of TMP-SMX for primary prophylaxis and treatment of TE in patients with HIV infection is consistent with the available data. | Current evidence fails to identify one superior regimen in terms of relative efficacy or safety for the treatment of HIV-associated cerebral toxoplasmosis. Use of TMP-SMX as preferred treatment is consistent with the available evidence and other real-world considerations. |
- CI, confidence interval; RR, risk ratio; TE, toxoplasmic encephalitis; RCT, randomized controlled trial; TMP-SMX, trimethoprim plus sulfamethoxazole; P-S, pyrimethamine plus sulfadiazine; P-C, pyrimethamine plus clindamycin; D-P, dapsone plus pyrimethamine; GI, gastrointestinal.
As only a limited number of randomized trials have been performed on this topic, we aimed to evaluate the treatment regimens for HIV-associated cerebral toxoplasmosis in a larger population by including comparative cohort studies. Our results are in congruence with those of the two previous reviews, confirming that treatment with P-S had similar efficacy and safety to P-C or TMP-SMX. We found that only liver impairment was more frequent with P-S when compared with P-C. Thus, the available evidence fails to identify any one superior regimen for the treatment of cerebral toxoplasmosis.
Cerebral toxoplasmosis has been the most common central nervous system disease in patients with AIDS from both developed and developing countries 25-27. In the HAART era, the burden of cerebral toxoplasmosis was reduced particularly in developed countries and to a lesser extent in developing countries. The situation is more serious in scenarios with nonavailability of HAART. However, globally, cerebral toxoplasmosis continues to afflict patients for several reasons: 1 late HIV diagnosis; 2 noncompliance with HAART or preventive antimicrobial drugs; and 3 virological and immunological HAART failure 28. For these reasons, cerebral toxoplasmosis poses a serious challenge in the management of HIV-infected patients.
Early recognition and prompt treatment of acute symptoms during the initial phase of cerebral toxoplasmosis reduces the risk of neurological sequelae and mortality. Historically, the regimen containing P-S has been preferred, and for patients who have contraindications for sulfadiazine use, the common alternative was P-C 9, 29. Approximately 70–90% of patients treated with these regimens demonstrate clinical and/or radiological improvement 30. In clinical practice, however, treatment with P-S or P-C is beset with several problems, including adverse events, poor tolerability, complex posology, and the absence of parenteral formulations (a major problem in patients with alteration of mental status). In this context, evaluation of alternative treatments continues to be a challenge. In patients with no allergies to sulfa drugs, TMP-SMX has been considered as a potential alternative 9, 10. One of the limitations to more widespread use of TMP-SMX is the lack of clinician expertise with this regimen in settings wherein P-S and P-C are available. Although diverging from classical and “paradigmatic” therapeutic management choices is not easy, a critical review of evidence can be useful in this scenario. TMP-SMX is routinely used in several settings, particularly in developing countries when P-S or P-C is not available. Evidence from these settings is scarce, as demonstrated in our review, where eight of nine included studies were conducted in developed countries.
The ideal RCT to identify the optimal treatment of cerebral toxoplasmosis should compare TMP-SMX with P-S using a noninferior design, be multicentre and double-blinded, and have a robust sample size. Using clinical response (i.e. complete plus partial response) as the primary outcome, the results from Torre et al.'s 1998 trial 21 (clinical response: TMP-SMX arm = 78% and P-S arm = 81%), an alpha of 5% and a power of 90%, then 690 patients are required to be 90% sure that the upper limit of a one-sided 95% CI (or equivalently a 90% two-sided CI) will exclude a difference in favour of the P-S arm of more than 12% (i.e. the noninferiority limit). Major international efforts are necessary to conduct this study, particularly in developing countries.
Currently, potential advantages of TMP-SMX over P-S or P-C include: 1 the convenience of the lower pill burden and dosing frequency and the availability of intravenous formulations; current guidelines suggest an option of parenteral TMP-SMX usage as initial treatment in severely ill patients 9; 2 the availability of several generic TMP-SMX formulations with the consequent impact on cost-effectiveness and increased accessibility; 3 prevention of Pneumocystis jirovecii pneumonia, other bacterial infections, and malaria 9, 31; and 4 the convenience of use simplifying the early initiation of HAART, which is associated with increased survival of HIV-infected patients with most opportunistic diseases.
Our study has some limitations. First, the quality of included studies was moderate to good. Most of the RCTs did not use blinding of treatments, which is essential to reduce information bias. We also included cohort studies, which have a higher probability of bias than RCTs; however, cohort studies were analysed independently from RCTs. Secondly, the number of studies and the total sample size were low. This may have reduced the probability of finding significant results for both efficacy and safety outcomes. Also, we were not able to run separate analyses by complete or partial clinical and radiological responses. Finally, specific doses of antimicrobials and duration of treatments were heterogeneous across studies. However, the direction of effects across different treatments, designs, doses and durations of treatment were similar across studies.
Previous reviews indicated that the choice of therapy will often be directed by available therapy 6, 7. In our systematic review, the available evidence fails to identify a superior regimen in terms of relative efficacy or safety for the treatment of cerebral toxoplasmosis. Although current evidence does not allow for a definitive recommendation, use of TMP-SMX as a preferred treatment option in HIV-associated cerebral toxoplasmosis is consistent with the available data and associated practical issues. Larger comparative studies are needed, particularly in developing countries.
Acknowledgements
Conflicts of interest: None of the authors has a conflict of interest to disclose. PT is currently an employee of Avery Dennison Corporation.
Financial disclosure: No funding was obtained for this study.