Advances in treatments for acute ischemic stroke
BMJ 2025; 389 doi: https://doi.org/10.1136/bmj-2023-076161 (Published 07 May 2025) Cite this as: BMJ 2025;389:e076161- 1Department of Neurology, Yale School of Medicine, New Haven, CT, USA
- 2Department of Neurology, Temple University Hospital, Philadelphia, PA, USA
- Correspondence to: R Sharma richa.sharma{at}yale.edu
ABSTRACT
Acute ischemic stroke is a leading global cause of death and disability. Intravenous thrombolysis was the first acute treatment developed for ischemic strokes. First with alteplase and now with tenecteplase, intravenous thrombolysis has remained a cornerstone of acute ischemic stroke management. In large vessel occlusions, endovascular thrombectomy has become the standard of care in acute stroke management for anterior and posterior circulation strokes. The boundaries for these treatments have expanded, which has improved outcomes in patients who were previously ineligible. This review summarizes the latest advances in interventions for acute ischemic stroke, extending beyond existing guidelines and review articles to explore emerging strategies and treatments currently under investigation.
Introduction
Acute ischemic stroke is defined as an interruption of blood supply to a region of the brain, and its cause varies. Regardless of its mechanism, an ischemic stroke often results in significant neurological deficits, and, if enough territories or critical regions are deprived of perfusion, in death. The global disease burden and mortality and morbidity associated with stroke have decreased slowly since the early 20th century.1 Although the drivers of this trend are multifactorial, a key aspect is the advent of evidence based treatments that, when delivered within the acute period in eligible patients, can prevent significant stroke related disability and death. These proven treatments include intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT), and the indications for both are increasingly expanding with emerging studies. Access to these effective treatments has transformed stroke systems of care worldwide, although their availability is still not universal. In this review, we summarize the evidence about the latest advances in the field of acute ischemic stroke, recent guidelines, and emerging treatments.2
Sources and selection criteria
We searched PubMed to identify systematic reviews, meta-analyses, randomized clinical trials, and large cohort studies examining acute treatments and interventions for acute ischemic stroke published in English between 1 January 2015 and 1 September 2024. Case reports and case series were not considered. Keyword terms used were “thrombectomy,” “endovascular procedures,” “thrombolytic therapy,” “tissue plasminogen activator,” “brain ischemia,” “extended window in acute stroke treatment,” “adjunctive therapy in acute stroke treatment,” “unknown onset in acute stroke treatment,” “access to acute stroke treatment,” and “contraindications in acute stroke treatment.” We also identified a descriptive epidemiological report published in 2024 by the American Heart Association/American Stroke Association to inform our discussion of stroke epidemiology.1Studies published before 2015 were included if relevant and for historical context.
Epidemiology
In 2021, 69.93 million people worldwide had an ischemic stroke, representing a 1.33% decrease since 2010.1 Regionally, the highest prevalence was seen in southern sub-Saharan Africa.1 In 2021, 7.44 million deaths caused by ischemic stroke were recorded, which represented a 17.39% decrease since 2010, with the highest mortality seen in Oceania and South East Asia.1 Although the absolute number of ischemic strokes increased by 70% since 1990, potentially owing to the increase in the world’s population, the incidence decreased by 17% between 1990 and 2019, likely because of decreased prevalence of stroke risk factors such as hypertension and diabetes.1 Stroke affects patients disproportionately. Compared with men, women have a higher lifetime risk of and increased mortality from stroke, regardless of age. In the United States, black people have a higher risk of stroke and are less likely to undergo thrombectomy compared with white people.1 Large vessel and small vessel atherosclerosis were more frequently associated causes of stroke in high income countries, whereas other or undetermined causes of stroke were seen more frequently in middle and low income countries.1 Risk factors for stroke are also disproportionate. For example, diabetes and hypertension are more commonly found in black and Asian people, and weight gain is associated with higher risk of stroke in women than in men.1
Intravenous thrombolysis
Since the landmark two part randomized controlled trial (National Institute of Neurological Disorders and Stroke (NINDS), n=624) showing the efficacy of alteplase (tissue plasminogen activator—tPA) as an acute stroke treatment to improve clinical outcomes at three months (39% v 26%, odds ratio 1.7, 95% confidence interval 1.1 to 2.6) and a 2014 meta-analysis of nine earlier alteplase trials confirming the benefit of intravenous thrombolysis in acute ischemic stroke if given within 4.5 hours of last known well (LKW; 35.3% v 30.1%, 1.26, 1.05 to 1.51), IVT has become the guideline based treatment for acute stroke within 4.5 hours of onset.3456 Tenecteplase, with its higher specificity for fibrin, longer half life, and lower risk of systemic hemorrhage, has since been developed and replaced tPA in many stroke centers.789 However, the boundaries for IVT administration continue to expand.
Unknown time of onset
A 2018 randomized controlled trial (WAKE-UP, n=503) showed an improvement in functional outcome at 90 days in patients with unknown LKW who were selected to receive thrombolysis versus placebo based on diffusion weighted imaging–fluid attenuated inversion recovery mismatch, a biomarker indicating that the stroke might have occurred within the last 4.5 hours (53.3% v 41.8%, odds ratio 1.61, 95% confidence interval 1.09 to 2.36).10 This finding was contested by a 2020 multicenter, randomized controlled trial in Japan (THAWS, n=131), which showed that while it was safe, administering tPA versus placebo in patients with stroke with diffusion weighted imaging–fluid attenuated inversion recovery mismatch had no effect on functional outcome at 90 days (47.1% v 48.3%, relative risk 0.97, 95% confidence interval 0.68 to 1.41).11 A multicenter, randomized controlled trial (TWIST, n=578) in 2023 attempted to identify patients eligible to receive tenecteplase among those who were within 4.5 hours of waking up with stroke symptoms using non-contrast computed tomography (CT) alone; although tenecteplase administration versus placebo was safe, there was no difference between the two when measuring functional outcome at 90 days (odds ratio 1.18, 95% confidence interval 0.88 to 1.58).12
Extended window
The first evidence of the potential benefit of IVT outside the 4.5 hour window was shown in 2012 (IST-3).13 In 2019, a randomized controlled trial (EXTEND, n=225) used CT perfusion criteria of penumbra-core ratio greater than 1.2 and with absolute difference greater than 10 mL and core less than 70 mL to select candidates for IVT up to nine hours after LKW. Although there was a modest benefit in the IVT group compared with placebo for good functional outcome at 90 days (35.4% v 29.5%, risk ratio 1.44, 95% confidence interval 1.01 to 2.06), a sixfold increase in intracerebral hemorrhage was seen (6.2% v 0.9%, 7.22, 0.97 to 53.5).14 A 2019 meta-analysis (total n=414), examining the pooled data from EPITHET, EXTEND, and ECASS 4-EXTEND, further provided support for administering IVT versus placebo for carefully selected patients in the 4.5-9 hour window for improved functional outcome at 90 days (36% v 29%, combined odds ratio 1.86, 95% confidence interval 1.15 to 2.99).15
A 2024 randomized controlled trial (TIMELESS, n=458) similarly showed that there were no safety issues with tenecteplase given up to 24 hours of LKW in patients with small core and large penumbra on the perfusion imaging, though there was no effect on median modified Rankin scale (mRS) score at 90 days for tenecteplase versus placebo (3 v 3, odds ratio 1.13, 95% confidence interval 0.82 to 1.57).16 The finding of this study is likely confounded by most of the trial’s patients having undergone thrombectomy. By contrast, when thrombectomy was unavailable, a 2024 randomized controlled trial (TRACE-III, n=516) showed that tenecteplase administration between 4.5 and 24 hours versus placebo was associated with improved functional outcome at 90 days (33.0% v 24.2%, risk ratio 1.37, 95% confidence interval 1.04 to 1.81) with no difference in mortality or symptomatic intracerebral hemorrhage.17
Alternative thrombolysis agents
Besides tPA and tenecteplase, alternative thrombolytic agents continue to be studied (table 1). A 2015 randomized controlled trial (DIAS-3, n=492) compared desmoteplase, which has a higher fibrin specificity than alteplase, and placebo in patients with stroke caused by an occlusion or high grade stenosis of a major intracranial artery within three to nine hours of symptom onset. Although safe, there was no difference in good clinical outcome at 90 days between the two groups (51% v 50%, odds ratio 1.20, 95% confidence interval 0.79 to 1.81). Non-immunogenic staphylokinase, which had been shown to result in higher reperfusion rates with fewer hemorrhagic complications than alteplase and tenecteplase in patients with myocardial infarction, was compared with alteplase in a 2021 randomized controlled non-inferiority trial of 385 patients with stroke within 4.5 hours of symptom onset. No differences in death or hemorrhagic complications were shown, and the difference in favorable outcome was 9.5% (95% confidence interval −1.7 to 20.7). As the lower limit of the 95% confidence interval did not cross the non-inferiority margin on 16%, the criterion for non-inferiority was not met.18
Different intravenous thrombolytic agents with their dosing regimen and advantages compared with alteplase. Alteplase and tenecteplase are the only agents currently in clinical use
With similar potential benefit and safety profile to the previous two agents, recombinant human prourokinase was compared with alteplase in a randomized controlled non-inferiority trial (PROST) in 2023 in 663 patients with stroke within 4.5 hours of symptom onset. At 90 days, 65.2% in the recombinant human prourokinase group and 64.3% in the alteplase group achieved good functional outcome (risk difference 0.89, 95% confidence interval −6.52 to 8.29), which was within the non-inferiority margin. The rates of symptomatic intracerebral hemorrhage were similar in both groups, but the recombinant human prourokinase group had less systemic bleeding.19 A 2024 randomized controlled non-inferiority trial (RAISE) compared reteplase, a recombinant plasminogen activator administered in two boluses of standardized dosing, and alteplase in 1412 patients with stroke within 4.5 hours of symptom onset. Good functional outcome at 90 days was seen in 79.5% v 70.4% (95% confidence interval 1.05 to 1.21), exceeding the non-inferiority criterion and showing potential superiority of reteplase, though any intracranial hemorrhage (7.7% v 4.9%, risk ratio 1.59, 95% confidence interval 1.00 to 2.51) and adverse events (91.6% v 82.4%, 1.11, 1.03 to 1.20) were higher with reteplase.
Adjunctive agents
Some studies have explored IVT in combination with other agents to enhance thrombolysis. Edaravone, a free radical scavenger shown to reduce injuries to the vascular endothelium, was administered early or late along with alteplase in patients with M1 or M2 occlusions within 4.5 hours of stroke onset in a 2016 randomized controlled study in Japan (YAMATO, n=165). No differences were reported in rates of early recanalization (within 1.5 hours after alteplase administration; 53% v 53%, P=1.000) or symptomatic intracerebral hemorrhage (5% v 2%, P=0.443) between the early and late edaravone groups.20 Argatroban, a direct thrombin inhibitor, has also been studied as an adjunctive therapy to IVT. Ninety patients receiving tPA but not undergoing thrombectomy were randomized to placebo, low dose argatroban, or high dose argatroban in a 2017 randomized controlled trial (ARTSS-2). At 90 days, good clinical outcome was achieved by 21% in the placebo group, 30% in the low dose argatroban group, and 32% in the high dose argatroban group. The relative risks for low dose, high dose, and either low or high dose argatroban were 1.17 (95% confidence interval 0.57 to 2.37), 1.27 (0.63 to 2.53), and 1.34 (0.68 to 2.76), respectively, showing no benefit of adjunctive argatroban.21 In 2023, ARAIS (n=817), a randomized controlled trial comparing argatroban with placebo in patients with stroke receiving tPA, showed similar lack of difference in good clinical outcome at 90 days (63.8% v 64.9%, risk ratio 0.98, 95% confidence interval 0.88 to 1.10).22 Argatroban and eptifibatide, a rapid glycoprotein IIb/IIIa inhibitor, were studied in patients with stroke who received IVT in a 2024 randomized controlled trial (MOST, n=514). No difference was reported in the mean utility mRS score at 90 days between argatroban and placebo (5.2 v 6.8; posterior P=0.002) and between eptifibatide and placebo (6.3 v 6.8; posterior P=0.041).23 To date, no beneficial adjunctive treatment to IVT has been identified.
IVT in patients with traditional contraindications
Derived from the criteria developed in the early days of tPA trials in acute ischemic strokes, IVT guidelines today have exclusion criteria that mainly center around reducing the risk of hemorrhagic complications.34 However, growing evidence shows that these criteria might be too restrictive. For example, a 2020 meta-analysis of six clinical trials (total n=52 823 across six studies) showed no increased risk of hemorrhagic transformation (combined odds ratio 1.48, 95% confidence interval 0.50 to 4.38), symptomatic hemorrhagic transformation (0.47, 0.09 to 2.55), or early mortality (0.60, 0.11 to 3.43) with IVT in patients who had taken a direct oral anticoagulant within 48 hours.24 Similarly, a 2023 retrospective cohort study (n=33 207) showed that among patients with stroke who received IVT and had taken a direct oral anticoagulant within 48 hours, the risk of symptomatic intracerebral hemorrhage was lower compared with no anticoagulation (odds ratio 0.57, 95% confidence interval 0.36 to 0.92).25
A 2020 retrospective observational study of 293 patients showed that while those with previous ischemic stroke within three months were less likely to be discharged home or have good functional outcome at discharge, the increased risk of symptomatic intracerebral hemorrhage after tPA was only seen in those with a history of ischemic stroke within the last 14 days (16.3% v 4.8%, odds ratio 3.7, 95% confidence interval 1.62 to 8.43).26 A 2022 systematic review of 23 studies (n=495) suggested that the presence of benign, as opposed to malignant or metastatic, intracranial tumors was not associated with increased risk of intracerebral hemorrhage after tPA (odds ratio 0.72, P=0.16 for risk of intracerebral hemorrhage after IVT in benign brain tumors; odds ratio 2.33, P<0.001 in malignant brain tumors).27 Although additional studies are required, the current literature seems to point to a reduction of the exclusion criteria for IVT.
Existing absolute contraindications for intravenous thrombolysis according to American Heart Association/American Stroke Association
Unknown time of onset or unwitnessed symptom onset with last known well >4.5 hours*
Awoke with symptoms with last known well >4.5 hours*
Extensive hypoattenuation on computed tomography scan
History of intracerebral hemorrhage
History of ischemic stroke within three months*
Severe head trauma within three months
Intracranial or intraspinal surgery within three months
Subarachnoid hemorrhage
Gastrointestinal malignancy
Gastrointestinal bleed within 21 days
Coagulopathy (international normalized ratio >1.7, activated partial thromboplastin time <40 seconds, prothrombin time >15 seconds)
Therapeutic low molecular weight heparin within 24 hours
Thrombocytopenia (platelet count <100 000/mm3)
Concurrent use of glycoprotein IIb/IIIa receptor inhibitors
Direct thrombin inhibitors or factor Xa inhibitors within 48 hours*
Infective endocarditis
Aortic arch dissection
Intra-axial intracranial neoplasm*
*Contraindications for which evidence supports use of intravenous thrombolysis despite their presence328
Systems of care designed to expand access to thrombolysis
Although the maximal benefit of thrombolysis was seen within the first 90 minutes of stroke onset in the initial NINDS trial, a 2016 review of the Get With The Guidelines-Stroke data showed that IVT started within 60 minutes of stroke onset was associated with increased odds of being able to be discharged home (odds ratio 1.25, 95% confidence interval 1.07 to 1.45), ambulating independently at discharge (1.22, 1.03 to 1.45), and freedom from disability (1.72, 1.21 to 2.46) compared with administration at later time points.529 Telestroke has the potential to hasten IVT administration. Telestroke has been in existence since 1999 and has been shown to be superior to telephone consults with neurologists in making accurate decisions about administering IVT.3031 More recently, a 2020 retrospective cohort study (n=12 803) showed that with every 10 telestroke consults done at a community hospital, there was a 1.8 minute decrease in the door-to-needle time (P=0.02).32
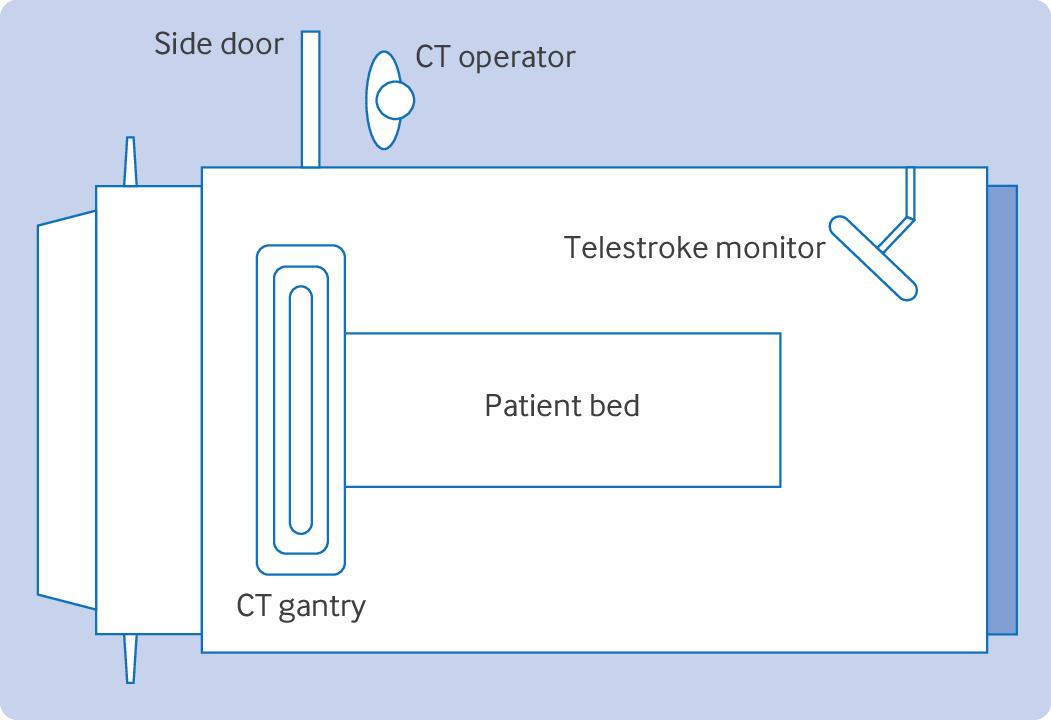
Mobile stroke units (MSUs) have also been used to optimize access to intravenous thrombolytics in stroke care (fig 1). MSUs are specialized ambulances with a CT scanner, emergency medical services personnel, a nurse, a radiology technician, and a neurologist—available in person or through telestroke, with capabilities to administer intravenous thrombolytics.3334 A 2014 German randomized controlled trial (PHANTOM-S, n=6182) showed that stroke care through MSUs decreased alarm-to-treatment time by 25 minutes without increased risk of intracerebral hemorrhage compared with controls (95% confidence interval 20-29 minutes).35 In 2021, a multicenter, prospective, observational study (BEST-MSU, n=1515) showed that stroke care through MSUs not only decreased mean time from stroke onset to IVT by 33 minutes, it also improved functional outcome at 90 days (utility weighted mRS score 0.73 v 0.67, odds ratio 2.12, 95% confidence interval 1.54 to 2.93).36 The same year, another prospective, observational study from Germany (B_PROUD, n=1543) showed a similar finding that patients with stroke treated by MSUs had lower median mRS score at three months compared with controls (1 v 2, odds ratio 0.71, 95% confidence interval 0.58 to 0.86).37 Despite potential benefits of MSUs, barriers for widespread adoption still exist, including high cost of purchase and maintenance, availability of specialized personnel, limited funding sources, and lack of recognition by governmental agencies.34
Layout of a typical mobile stroke unit. CT=computed tomography
Minor strokes
Evidence exists that IVT might not even be necessary in some patients with stroke. Although it was terminated early because of slow recruitment, a 2018 randomized controlled trial (PRISMS, n=313) showed that there was no difference in favorable outcome at 90 days between alteplase administered within three hours of symptom onset compared with oral aspirin alone in patients with minor strokes, defined as National Institutes of Health Stroke Scale ≤5 (78.2% v 81.5%, risk difference −1.1%, 95% confidence interval −9.4% to 7.3%).38 This study was followed by another randomized controlled trial in 2023 (ARAMIS, n=760), which showed no difference between 12 days of dual antiplatelet therapy with aspirin and Plavix, followed by aspirin monotherapy, and alteplase administered within 4.5 hours of symptom onset in patients with similarly defined minor stroke (93.8% v 91.4%, risk difference 2.3%, 95% confidence interval −1.5% to 6.2%).39
Mechanical thrombectomy
In 2015, through careful selection of patients with proximal anterior circulation large vessel occlusions—mostly targeting intracranial internal carotid artery and M1—several randomized clinical trials (MR CLEAN, EXTEND-IA, ESCAPE, SWIFT PRIME, and REVASCAT) individually showed the benefit of EVT plus tPA versus tPA alone in the early window of less than six hours from stroke onset.4041424344 A 2016 meta-analysis of the five trials (HERMES, total n=1287) came to a similar conclusion—compared with controls, EVT was more likely to lead to good clinical outcome at 90 days (combined odds ratio 2.49, 95% confidence interval 1.76 to 3.53), with a number needed to treat of 2.6 for EVT to improve disability by one mRS category.45 The window for EVT was extended up to 24 hours from stroke onset in 2018 when two randomized controlled trials (DAWN and DEFUSE 3) showed the benefit of EVT in patients with clinical deficit-ischemic core mismatch between 6 and 24 hours after LKW using perfusion imaging.4647 Selecting patients based on collateral flow on CT angiography was also shown to be effective in the 6-24 hour window.48 Since the initial anterior circulation EVT trials, additional studies have been published to include even more patients and techniques.
Posterior circulation
It is important to note that while the early successful EVT trials focused on the anterior circulation, infarcts in the posterior circulation still represent about 20% of all ischemic strokes and are associated with significant morbidity and mortality.495051 In 2020, a randomized open label trial in China (BEST, n=131) found no difference in mRS score at 90 days in patients with vertebrobasilar occlusions who underwent EVT plus medical treatment versus medical treatment alone within eight hours of symptom onset (42% v 32%, odds ratio 1.74, 95% confidence interval 0.81 to 3.74).52 In 2021, another randomized controlled trial (BASICS, n=300) narrowed the scope to only include patients for whom EVT could be performed within six hours of stroke onset but did not show any significant difference in the 90 day functional outcome between the EVT group and the medical management group (44.2% v 37.7%, risk ratio 1.18, 95% confidence interval 0.92 to 1.50).53 Although these were negative trials, owing to poor recruitment, both studies had large numbers of patients treated outside of trial protocols; additionally, BEST was prematurely terminated because of high crossover rates.54
In 2022, two randomized controlled trials (ATTENTION, n=340; BAOCHE, n=217) showed that EVT for basilar occlusions when performed within 12 and 24 hours of stroke onset, respectively, increased the chance of good clinical outcome at three months twofold (for ATTENTION, risk ratio 2.06, 95% confidence interval 1.46 to 2.91, number needed to treat=4; for BAOCHE, 1.81, 1.26 to 2.60, 4.5).5556 The meta-analysis of the four posterior circulation EVT trials confirmed the benefit of EVT in posterior circulation large vessel occlusions (pooled risk ratio 1.83 for mRS score 0-2 at 90 days, 95% confidence interval 1.08 to 3.08), despite a higher chance of symptomatic intracerebral hemorrhage (risk ratio 7.77, 95% confidence interval 2.36 to 25.59).57 Complementing the anterior circulation trials, these posterior circulation EVT trials further solidify the role of EVT in acute ischemic stroke treatment.
Adjunctive treatments with mechanical thrombectomy
Although there are some conflicting data, the benefit of bridging therapy with IVT for eligible patients before EVT has been shown in several trials.58596061626364 Alternative agents have yet to show any benefit. Nerinetide, an eicosapeptide that interferes with postsynaptic density protein 95 and leads to neurotoxicity signal inhibition, was not shown to have any effect on good clinical outcome at 90 days when administered within 12 hours of stroke symptom onset after EVT in a randomized controlled trial (ESCAPE-NA1, n=1105; 61.4% v 59.2%, risk ratio 1.04, 95% confidence interval 0.96 to 1.14).65 Intravenous tirofiban, a selective glycoprotein IIb/IIIa inhibitor with a short half life, administered before EVT, also did not have any effect on good functional outcome at 90 days in another randomized controlled trial (RESCUE BT, n=948; median mRS score at 90 days 3 v 3, odds ratio 1.08, 95% confidence interval 0.84 to 1.36).66
Large core infarcts
In addition to posterior circulation stroke, EVT has been explored as a therapeutic option in ischemic stroke with large infarct burden. The earlier trials focused on smaller infarct sizes—less than 70 mL of ischemic core on perfusion imaging or infarcts involving less than a third of the middle cerebral artery territory.4647 HERMES showed that a larger ischemic core volume was associated with decreased odds of achieving functional independence.45 However, a secondary analysis of SELECT (prospective cohort, n=105, EVT 31% v medical treatment 14%, odds ratio 3.27, 95% confidence interval 1.11 to 9.62) and a 2018 meta-analysis (for patients with >33% middle cerebral artery involvement, odds ratio 1.70, 95% confidence interval 1.04 to 2.78) showed that despite lower odds of good clinical outcome, EVT in large core infarcts is still associated with better outcome than medical treatment alone.6768 In 2022, a randomized controlled trial (RESCUE-Japan LIMIT, n=203) found that in patients with large core strokes, defined as Alberta Stroke Program Early Computed Tomography Score (ASPECTS) 3-5, those who underwent EVT were more than twice as likely to achieve good functional outcome at 90 days compared with controls (risk ratio 2.43, 95% confidence interval 1.35 to 4.37).69
In 2023, another randomized clinical trial (SELECT2, n=252) found that patients with large ischemic infarct, defined as ASPECTS 3-5 or ischemic core volume greater than 50 mL, who underwent EVT had higher likelihood of achieving functional independence at 90 days with similar mortality rates compared with controls (odds ratio 1.51, 95% confidence interval 1.20 to 1.89).70 Published in the same year, a randomized control trial from China (ANGEL-ASPECTS, n=456) included patients with even larger ischemic core, defined as 70-100 mL, and showed similar benefit to EVT (odds ratio 1.37, 95% confidence interval 1.11 to 1.69).71 These findings were consistent in 2024 randomized clinical trials TENSION (n=253, EVT v medical treatment for median mRS score at 90 days, odds ratio 2.39, 95% confidence interval 1.47 to 3.90) and LASTE (n=333, EVT v medical treatment for median mRS score at 90 days, 1.63, 1.29 to 2.06).7273 Offering EVT in patients with large core infarcts might soon become standard practice.
Medium vessel occlusions
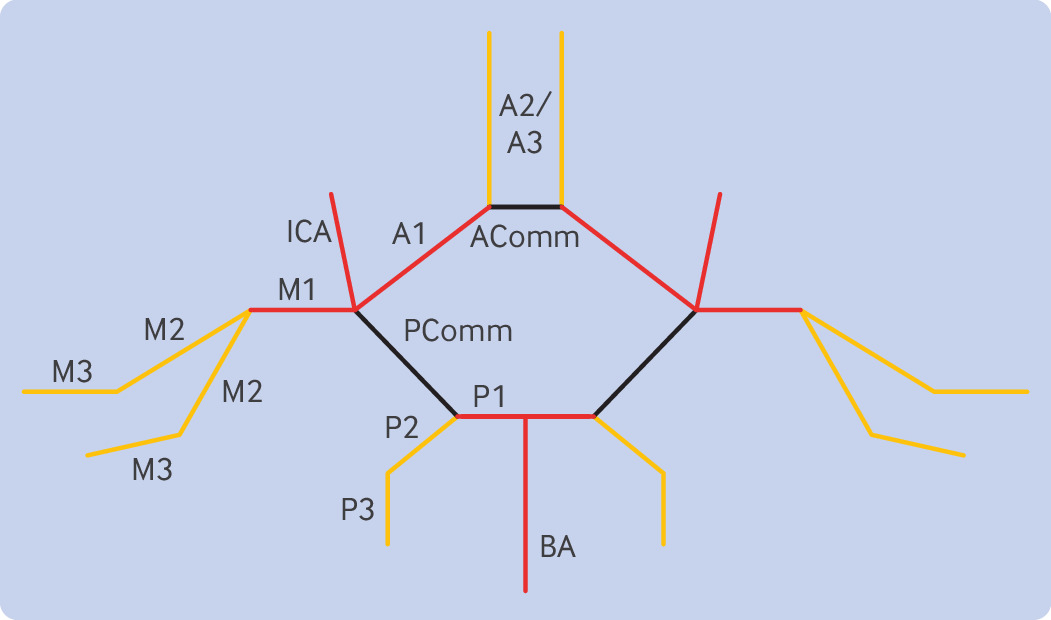
Despite a wealth of evidence for EVT in large vessel occlusions, evidence for the benefit of EVT in medium sized vessel occlusions (MeVOs) is lacking. A medium sized vessel is defined as M2 and M3 segments of the middle cerebral artery, A2 and A3 segments of the anterior cerebral artery, and P2 and P3 segments of the posterior cerebral artery (fig 2).74 Although MR CLEAN and EXTEND-IA included patients with large vessel occlusions in M2, HERMES concluded that there was no benefit or harm in performing EVT for M2 occlusions; however, this MeVO only represented 51 patients or 8% of the patients included in the meta-analysis.404145 Medium sized vessels also have thinner walls, increasing the risk for potential procedural complications, and MeVOs might be associated with less severe deficits compared with large vessel occlusions.75 A 2024 retrospective cohort study (DUSK, n=321) showed no difference in good outcome (odds ratio 1.32, 95% confidence interval 0.97 to 1.80) or mortality (1.20, 0.78 to 1.86) at 90 days in patients who underwent EVT versus medical treatment alone.76 Regardless, most physicians consider MeVOs—specifically M2 occlusions—a viable target for EVT.77 Additional clinical trials, including DISCOUNT, DISTAL, DISTALS, and ESCAPE-MeVO, are currently recruiting patients with MeVO for EVTs.78
Locations of large vessels (red) and medium sized vessels (orange). AComm=anterior communicating artery; BA=basilar artery; ICA=internal carotid artery; PComm=posterior communicating artery
Acute stenting of cervical internal carotid artery
Unlike a typical symptomatic external carotid artery stenosis, which is best revascularized in the two week window after the stroke, a tandem occlusion of a symptomatic carotid artery and an ipsilateral intracerebral vessel might benefit from an emergent carotid intervention.79 A 2019 multicenter, observational study (the TITAN registry, n=205) found that emergent carotid stenting combined with mechanical thrombectomy for tandem occlusions can safely be performed despite the use of IVT; in fact, previous IVT was associated with significantly lower all cause mortality at 90 days (8% v 20%, P=0.017).80 Also in 2019, the same analysis of the STRATIS registry (n=147) did not show similar effect of IVT in this patient population; however, it did show that those who underwent simultaneous carotid stenting with intracranial thrombectomy had higher rates of good clinical outcome at 90 days than those who had thrombectomy alone (68.5% v 42.2%, P=0.003).81
A 2022 meta-analysis of nine studies showed that carotid stenting was associated with greater odds of reperfusion (combined odds ratio 1.89, 95% confidence interval 1.26 to 2.83) and good functional outcomes at three months (1.95, 1.24 to 3.05) compared with balloon angioplasty in internal carotid artery and middle cerebral artery tandem occlusions.82 A similar benefit was shown in a 2023 meta-analysis of 46 studies comparing emergent carotid stenting plus thrombectomy versus thrombectomy alone (1.52, 1.19 to 1.95), though there was also a higher risk of symptomatic intracerebral hemorrhage (1.97, 1.23 to 3.15).83 However, a prospective observational study in 2023 concluded that the rates of symptomatic intracerebral hemorrhage were similar in these two groups (odds ratio 0.90, 95% confidence interval 0.46 to 2.40).84 The observational data so far seem to suggest that acute carotid stenting in the setting of tandem occlusions is beneficial. PICASSO, CASES, and EASI-TOC are currently under way and studying this question.
Rescue stenting of intracranial artery
Although previous trials failed to show the benefit of intracranial stenting as secondary prevention of strokes caused by ipsilateral intracranial atherosclerosis, emergent intracranial stenting in the setting of failed EVT is a potential treatment option.8586 According to a single center, retrospective study of 596 patients, about 20% of EVTs failed for various reasons, including device failure and presence of arteriopathy.87 In patients like these, a 2022 retrospective cohort study (SAINT, n=499) found that rescue intracranial stenting of a large intracranial vessel after a failed thrombectomy compared with no rescue resulted in a favorable shift in the overall mRS distribution (odds ratio 2.31, 95% confidence interval 1.61 to 3.32), higher rates of functional independence (35.1% v 7%, odds ratio 6.33, 95% confidence interval 3.14 to 12.76), and lower mortality rate at 90 days (28% v 46.5%, 0.55, 0.31 to 0.96), with similar rates of symptomatic intracerebral hemorrhage (7.1% v 10.2%, 0.99, 0.42 to 2.34).88 Several meta-analyses found similarly favorable results for rescue stenting, showing recanalization rates of more than 80%—likely one of the main reasons for clinical improvement in these patients.8990 More studies are needed, however, before this strategy is adopted in clinical practice.
Blood pressure management
In patients receiving IVT, the blood pressure goal is <185/110 mm Hg before administration and <180/105 mm Hg after treatment based on the initial NINDS study.391 In 2019, a partial factorial, open label, blinded endpoint trial (ENCHANTED, n=2227) investigated whether intensive blood pressure control after intravenous thrombolysis was beneficial. The trial showed that lowering the systolic blood pressure to 130-140 mm Hg within one hour of IVT did not result in any improvement in 90 day functional outcome compared with those with systolic blood pressure <180 mm Hg (odds ratio 1.01, 95% confidence interval 0.87 to 1.17). However, there were significantly fewer occurrences of any intracerebral hemorrhage or hemorrhagic transformation associated with the lower blood pressure target (14.8% v 18.7%, odds ratio 0.75, 95% confidence interval 0.60 to 0.94).92
Similar to after IVT, the blood pressure target is set at <180/105 mm Hg after EVT according to the existing guidelines, although the level of evidence for this recommendation is weak.391 A prospective observational study in 2017 (n=88) showed that those who were functionally independent at three months had lower systolic blood pressure in the first 24 hours after EVT (160±19 v 179±23 mm Hg, P=0.001).93 Another prospective, observational study in 2020 (n=90) used a tailored blood pressure goal determined through near infrared spectroscopy derived tissue oxygenation to maximize preservation of autoregulation, rather than a fixed goal, after EVT. The study showed that the proportion of time spent in mean arterial pressure above the upper limit of autoregulation was associated with worse 90 day outcome (odds ratio per 10% 1.84, 95% confidence interval 1.3 to 2.7).94 By contrast, a 2021 randomized controlled trial (BP-TARGET, n=324) showed that intensive blood pressure control (100-129 mm Hg) within one hour and maintained for 24 hours after EVT did not reduce the rate of radiographic intracerebral hemorrhage on follow-up imaging in 24-36 hours compared with standard care (130-185 mm Hg; odds ratio 0.96, 95% confidence interval 0.60 to 1.51), with similar rates of hypotensive events for both.95
A 2022 randomized controlled trial (ENCHANTED2/MT, n=821) investigated functional outcomes at 90 days for intensive blood pressure management (<120 mm Hg, achieved within one hour of EVT, and sustained for 72 hours) and less intensive management (140-180 mm Hg), and showed that the intensive management was associated with greater poor functional outcome (odds ratio 1.37, 95% confidence interval 1.07 to 1.76) with no significant differences in symptomatic intracerebral hemorrhage.96 Similarly, a 2023 randomized controlled trial from South Korea (OPTIMAL-BP, n=306) showed that systolic blood pressure <140 mm Hg for 24 hours after EVT was associated with lower likelihood of functional independence at three months compared with standard care (140-180 mm Hg; 39.4% v 54.4%, odds ratio 0.56, 95% confidence interval 0.33 to 0.96).97 Also in 2023, another randomized controlled trial (BEST-II, n=120) showed no difference in stroke burden at 36 hours (follow-up infarct volume for each mm Hg decrease in the SBP target was −0.29 mL, 95% confidence interval −0.81 to ∞; futility P=0.99) or degree of disability at 90 days (utility weighted mRS score for each mm Hg decrease in systolic blood pressure −0.0019, 95% confidence interval −∞ to 0.0017; futility P=0.93) in patients in whom systolic blood pressure was lowered to various targets <180 mm Hg.98 Although not a new method, blood pressure management continues to be a critical part of acute ischemic stroke treatment.
MRI in acute stroke management
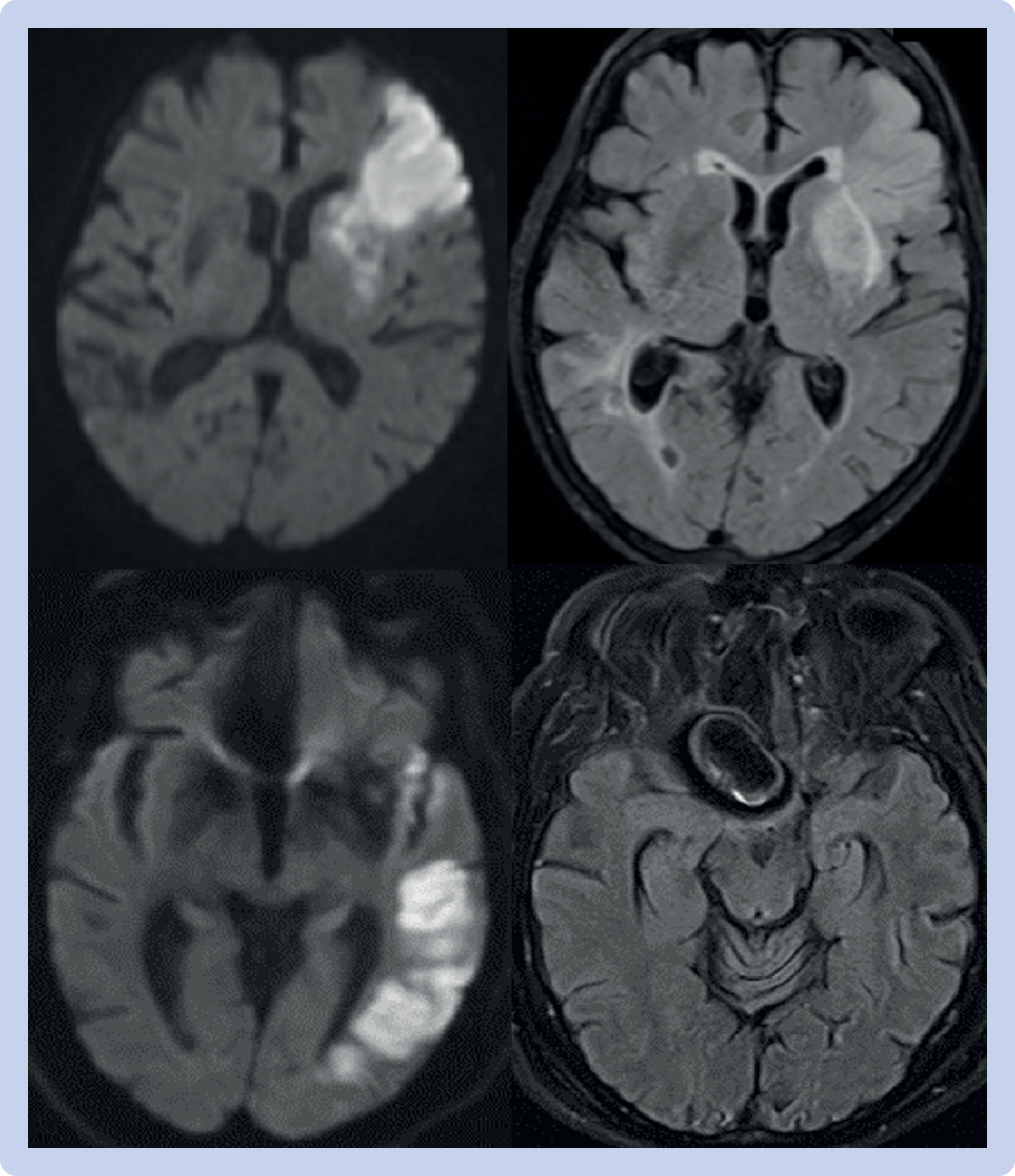
Magnetic resonance imaging (MRI) was used to identify eligible patients for earlier EVT trials and has been studied in identifying patients eligible for IVT in the setting of unknown time of onset. However, the use of MRI in acute clinical settings has been limited because of its sparse availability, greater resource utilization, and prolonged preparation for each scan. With the advent of hyperacute protocols, which can be completed within 15 minutes, MRI’s role in acute stroke management is being investigated.99 A small prospective cohort study (n=57) in 2016 showed that the use of hyperacute MRIs to identify patients with acute ischemic stroke among those thought to have stroke mimics did not affect the door-to-needle time (39 minutes (before hyperacute MRI) v 37 minutes (after hyperacute MRI), P=0.63) and symptomatic hemorrhage rates (4.5% v 1.9%, P=0.32).100 A 2018 retrospective cohort study (n=219) showed that it was feasible to use diffusion weighted and perfusion weighted MRIs alone to identify patients with large vessel occlusions who were eligible for EVT (96% were correctly identified; inter-rater reliability κ=0.938, P=0.855) and even correctly localize the segment of occlusion (96% were correctly identified; κ=0.922, P=0.817).101 Hyperacute MRIs could also potentially have cost advantages. A 2017 retrospective cohort study (n=267) showed that the average daily direct cost of caring for patients with stroke was reduced by 24.5% (95% confidence interval 14.1% to 33.7%, P<0.001) when comparing cost of care before and after the implementation of hyperacute MRI, without affecting the length of stay (10.6 v 9.9 days, P<0.42).102 Although more studies are needed, these findings underscore the growing potential of hyperacute MRIs to enhance acute stroke care (fig 3).
Comparisons of DWI and T2 FLAIR images in acute and hyperacute ischemic strokes without large vessel occlusion. Top: DWI hyperintensity in left frontal region (left), with corresponding T2 FLAIR hyperintensity (right), showing acute ischemic stroke. Bottom: DWI hyperintensity in left temporal region (left) without corresponding T2 FLAIR hyperintensity (right) from hyperacute MRI protocol. The patient with the bottom images would be considered a potential candidate for intravenous thrombolysis based on the MRI. DWI=diffusion weighted imaging; FLAIR=fluid attenuated inversion recovery; MRI=magnetic resonance imaging
Emerging treatments
Efforts continue to expand the time windows for acute stroke interventions. A 2023 retrospective cohort study (n=121) showed that, compared with those who underwent EVT within the 6-24 hour window, patients who underwent EVT beyond 24 hours tolerated the procedure with similar rates of symptomatic intracerebral hemorrhage (odds ratio 0.52, 95% confidence interval 0.19 to 1.44); yet, these patients were less likely to be functionally independent (0.24, 0.11 to 0.52) and had higher odds of mortality at 90 days (2.34, 1.13 to 4.84).103 With careful selection of patients using the eligibility criteria for the DAWN trial, another retrospective cohort study (n=128) showed similar rates of functional outcome (43% v 48%, P=0.68) and symptomatic intracerebral hemorrhage (5% v 6%, P=0.87) between EVT beyond 24 hours and EVT within the standard window.104 RESCUE END-LOW and TRACK-LVO Late are currently under way to study the impact of treatment beyond the 24 hour window. Given the clinical success of emergent stenting for failed thrombectomy in improving outcomes, RES-CAT is currently being devised to study this procedure in the setting of a randomized clinical trial.105 With the introduction of artificial intelligence into mainstream culture, this technology is also being deployed in acute stroke care. A 2024 cluster randomized trial (n=21603) showed that patients with acute stroke who were treated by physicians using an artificial intelligence clinical decision support system had fewer vascular events (2.9% v 3.9%, hazard ratio 0.75, 95% confidence interval 0.59 to 0.95) than controls.106 Neuroprotective agents are also being studied as an adjunctive therapy to EVT. A 2023 phase 1, randomized controlled trial (n=119) showed that administering ApTOLL, a DNA aptamer with potential anti-inflammatory effects through its antagonistic action on toll like receptor 4, was associated with a higher chance of good functional outcome at 90 days (odds ratio 2.44, 95% confidence interval 1.76 to 5.00) in patients who underwent EVT.107
Inequalities in administration of acute stroke treatments
Although not an advance in treatment, inequalities in acute ischemic stroke care must be recognized. A 2022 systemic review (30 studies, n=393 186) revealed that white patients were more likely to use emergency medical services than black, Asian, and Hispanic patients (white patients 59.8%, 95% confidence interval 56.5% to 63.1%; black patients 55.6%, 51.4% to 59.8%; Asian patients 54.7%, 47.9% to 61.5%; Hispanic patients 53.2%, 50.1% to 56.3%). The review also showed that white patients were more likely to arrive at an emergency department within three hours of stroke onset (white patients 37.5%, 27.7% to 48.4%; black patients 26.0%, 17.0% to 37.7%; Hispanic patients 28.9%, 25.3% to 32.9%), and that acute stroke interventions—in particular IVT—were more often used for white patients than non-white patients (white patients 2.8%, −5.2% to 10.8%; black patients 2.3%, −6.1% to 10.7%; Asian patients 2.3%, −15.5% to 20.0%; Hispanic patients 2.6%, −9.6% to 14.9%).108
A prospective cohort study in 2020 (n=2977) showed that this inequality between white and non-white patients persisted even in a telestroke environment (odds ratio 1.47, 95% confidence interval 1.17 to 1.84).109 Inequalities in acute stroke care are also seen across socioeconomic statuses. Patients of lower socioeconomic status had up to 30 minutes of additional delay between alerting the emergency medical services and time to brain CT scan (3 hours 47 minutes (lowest socioeconomic status) v 3 hours 17 minutes (highest socioeconomic status), P<0.05), perhaps owing to lower likelihoods of receiving the highest priority and recognition of a cerebrovascular event by the emergency medical services.110 Even after initial stroke care in hospital, those of lower socioeconomic status have higher risk of one year mortality than those of higher socioeconomic status (hazard ratio 1.77, 95% confidence interval 1.17 to 2.68), according to a longitudinal population based cohort study (n=3834).111 Additional studies are urgently needed to address healthcare inequalities in acute stroke care because advances in treatments alone cannot lead to improved outcomes without equitable care.
Guidelines
Several guidelines about the management of acute stroke have been recently published, including the American Heart Association/American Stroke Association (AHA/ASA) and the European Stroke Organisation (ESO) guidelines.34112113114115116 Although the AHA/ASA have not updated their guidelines since 2019, the ESO has maintained its guidelines up to date to incorporate newer evidence. The ESO makes recommendations for IVT between 4.5 and 9 hours from LKW based on MRI or CT perfusion, tenecteplase as an alternative to alteplase, the use of MSUs, and EVT for basilar artery occlusions, while the AHA/ASA guidelines do not provide specific recommendations for these treatments.4114115116 Neither the AHA/ASA nor ESO makes specific recommendations about adjunctive agents to IVT and EVT, EVTs in medium vessel occlusions and large core infarcts, acute stenting, and rescue stenting for failed EVT.
Conclusion
Acute ischemic stroke has evolved from a largely untreatable emergent condition to one with several evidence based interventions. Treatments now include potentially expanded eligibility for intravenous thrombolysis and mechanical thrombectomy, even in extended time windows and in patients with large core infarcts or posterior circulation strokes, respectively. Emerging thrombolytic agents, as well as acute and rescue stenting techniques, offer additional options for patients to have improved outcomes after stroke. As acute stroke care continues to advance, addressing inequalities in access to treatment and ensuring equitable care will be critical to achieving the full benefit of these advances.
Acknowledgments
The authors thank Victoria Helwig and Vermetha Polite for technical support and Melissa C Funaro, clinical research and education librarian, for help with the literature searches; and all individuals from the Cushing/Whitney Medical Library. The authors also acknowledge Mr Joseph Engel for his thoughtful review of this manuscript.
Patient involvement: This manuscript was reviewed by Mr Joseph Engel, an ischemic stroke survivor who was treated at Yale-New Haven Hospital. He has experienced first hand the long term symptoms associated with ischemic stroke. He encourages the use of evidence based therapies to abort an acute ischemic stroke in eligible patients to prevent post-stroke sequelae. He has provided feedback to strengthen this manuscript.
Footnotes
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: KL and RS both had equal responsibility in the planning, literature search and review, and drafting of the initial and subsequent revisions of the manuscript. KL and RS both gave the final approval of the submitted manuscript. KL and RS are the guarantors.
Competing interests: We have read and understood the BMJ policy on declaration of interests and declare the following interests: None.
Provenance and peer review: Commissioned; externally peer reviewed.