Vernal keratoconjunctivitis: a major review
Department of Ophthalmology
Mohammad Dossary Hospital
PO Box 335 Al Khobar 31952 Saudi Arabia Tel: +966 3 8936380
Fax: +966 3 8950735
Email: sunkaru79@hotmail.com
Abstract.
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral, at times asymmetrical, seasonally exacerbated, allergic inflammation of the ocular surface, involving tarsal and/or bulbar conjunctiva. Though the allergic nature of this entity has been accepted for a long time, the accumulation of a large amount of immunological data has proved that the pathogenesis of VKC is much more complex than a mere type 1 hypersensitivity reaction. In the past several years, many clinical and experimental studies about the cells and mediators involved in initiating and perpetuating the ocular allergic inflammation have shown that T helper type 2 cells and their cytokines, corneal fibroblasts and epithelium along with various growth factors play an important role in the pathogenesis of VKC. Based on this information about the pathogenesis of VKC newer, more selective drugs like anti-chemokine receptor antibodies and leukotriene receptor antagonists are under evaluation. Cyclosporine has been shown to be effective in the treatment of VKC but further randomized control trials are required to establish the minimum effective concentration.
Introduction
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral, at times asymmetrical, seasonally exacerbated, allergic inflammation of the ocular surface, involving tarsal and/or bulbar conjunctiva. It is more common in children and young adults having an atopic background. It was first mentioned in the ophthalmic literature as conjunctiva lymphatica more than 150 years ago. Subsequently, most of the doyens of ophthalmology during that period (Arlt, Dasmarres, von Graefe, Axenfeld, Trantas and Herbert) published about this interesting malady (Barney 1997). Different authors, at different times, described it as spring catarrh, phlyctenula pallida, circumcorneal hypertrophy, recurrent vegetative conjunctiva, verrucosa conjunctiva and aestivale conjunctiva, calling attention to the various aspects of this disease. Although the allergic nature of this entity has been accepted for a long time, its exact aetiology and pathogenesis is still unclear. Recently, many clinical and experimental studies about the cells and the mediators involved in initiating and perpetuating ocular allergic inflammation have broadened our knowledge about the pathophysiology of this disease. The accumulation of a large amount of immunological data has established that the pathogenesis of VKC is much more complex than a mere type 1 hypersensitivity reaction. To the present day, the precise role played by genetic predisposition and environmental factors in the onset, progression and resolution of this self-limiting, but at times incapacitating, childhood entity is an enigma. Despite the universal acceptance of the nomenclature vernal keratoconjunctivitis, occurrence of this disease is not limited to spring, with episodes of reactivity being quite common in the winter. The initial seasonal attacks turn into perennial disease after a few years. The efficiency of school-aged children decreased profoundly because of the chronic and recurrent course. Although this is not usually a blinding disease, visual impairment may occur if the cornea is involved.
Geographical distribution
VKC has a wide geographical distribution. Varying prevalence has been reported in different ethnic groups. Young males in dry and hot climates are primarily affected. It is more common in temperate zones of Mediterranean areas, central and west Africa, the Middle East, Japan, the Indian subcontinent and South America. VKC cases are also seen in Western Europe (including the UK and Sweden), Australia and North America – although the prevalence of VKC in these countries has probably increased because of migration of susceptible populations. After the recent decline of endemic trachoma, VKC is a leading cause of outpatient ophthalmic morbidity among Palestinians of east Jerusalem, the West Bank and Gaza (O’Shea 2000).
Demography
VKC usually starts before the age of 10 years. The earliest reported age of onset is 5 months (Ukponmwan 2003). It generally resolves after puberty, usually around 4–10 years after onset (Bielory 2000; Leonardi 2002a). The disease is more common among males, with the male to female ratio reported in the literature varying from 4 : 1 to 2 : 1 (Neumann et al. 1959; Beigelman 1965; Bonini et al. 2000). The male preponderance in VKC is conspicuous below 20 years of age but after 20 years, the male and female ratio becomes almost equal (Bielory 2000; Bonini et al. 2000). Positive staining for oestrogen and progesterone receptors in conjunctiva from VKC, predilection for male and resolution after puberty suggest the role of hormonal factors in the development of VKC (Bonini et al. 1995b).
Genetics and family history
So far, no genetic predisposing factor has been identified for VKC but the predominance of VKC in Asia and Africa, along with the persistence of this predilection in migrated African and Asian populations, strengthens the possibility of a genetic predisposition. VKC is more common among individuals of Asian and African origin living in Sweden (Montan et al. 1999). Although no genetic analysis has been performed to confirm a relationship between VKC and a particular genotype, the constant and increased presence of eosinophils in blood, tears and conjunctival scrapings, the expression of a multitude of mediators and cytokines, as well as the predominance of CD4 cells locally suggest that VKC may be a phenotypic model of upregulation of the cytokine gene cluster on chromosome 5q. The cytokine gene cluster, through its products like Interleukin (IL)-3, -4, -5 and granulocyte/macrophage-colony-stimulating factor (GM-CSF), regulate the prevalence of T helper cell type 2 (Th2), the growth and function of mast cells and eosinophils as well as the production of immunoglobulin (Ig) E in VKC (Bonini et al. 1995a). Family history of allergic disorders such as asthma, rhinitis, eczema, urticaria and multiple atopic diseases was reported in 49% of patients suffering from VKC (Bonini et al. 2000).
Associated conditions
Atopy, defined as the presence of allergen-specific IgE antibodies, is common among VKC patients. One third of VKC patients have multiple atopic diseases (Bonini et al. 2000). Atopy is less common in limbal compared to tarsal VKC (Tuft et al. 1989). Asthma is the most common atopic disease seen among VKC patients.
Fifteen per cent of VKC patients were reported to have keratoconus; 6% of these developed hydrops (Iqbal et al. 2003). A higher incidence of keratoconus and acute hydrops among VKC has been ascribed to excessive eye rubbing (Cameron et al. 1989). Acute hydrops with no history of eye rubbing and no hydrops, despite the history of frequent rubbing in many patients with keratoconus and VKC, suggest that acute hydrops may also be a result of a complex interaction between hereditary and environmental factors directed against the corneal endothelial in a susceptible population (Rehany & Rumelt 1995; Totan et al. 2001). Sex-hormone-related diseases such as gynaecomastia, polycystic ovary syndrome, mammary fibroadenoma, adiposogenital dystrophy and autoimmune diseases were reported by 2% of patients suffering with VKC (Bonini et al. 2000). In a gender- and age-matched study, a positive correlation between eyelash length and severity of VKC has been reported. It is postulated that long lashes may represent the protective mechanism against physical agents that might have an important role in the aetiopathogenesis of VKC, although the chemical mediator responsible for lash growth was not identified (Pucci et al. 2005).
Clinical features and diagnosis
In its typical form, VKC presents with pruritus, hyperaemia, photophobia and watering. VKC is bilateral in most (98%) patients, although minor differences in severity between the eyes are common (Bonini et al. 2000). Patients suffering from VKC may have several episodes of active inflammation throughout the year. Initially seasonal disease may become perennial after a few years. In approximately one quarter of VKC patients the disease smolders throughout the year, without any remission, from the onset (Bonini et al. 2000). Pruritus may vary from mild to intense and be exacerbated by exposure to wind, dust, bright light, hot weather or physical exertion associated with sweating. Exaggerated hyper-reactivity to non-specific stimuli such as heat, sun and wind during active and quiescent phases of VKC suggest neural involvement (Bonini et al. 1992a). Interestingly, Tabuchi et al. (2004) reported Staphylococcus aureus to be one of the exacerbating factors in VKC.
Thick mucus hyper-secretion with sticky mucous filaments, called ‘ropy discharge’, is a characteristic of VKC. Transient limbal or conjunctival yellow-white points or deposits, known as Horner–Trantas’s dots (Fig. 1) are degenerating eosinophils and epithelial cell debris. In a cohort of Asian patients suffering from VKC, perilimbal conjunctival pigmentation has been reported to be a constant finding. The extent of pigmentation did not correlate with the severity of symptoms and signs of VKC. The pigmentation persisted when the disease was inactive (Rao et al. 2004).
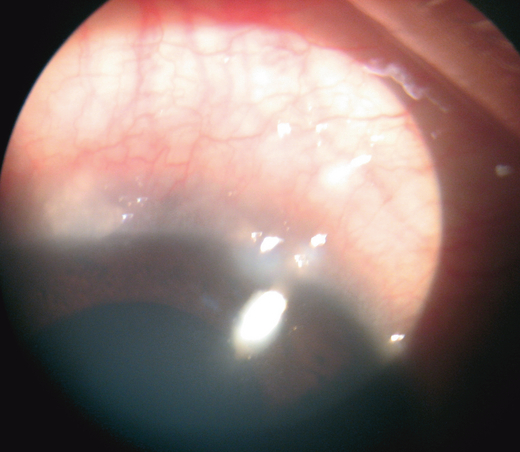
Horner-Tranta’s dots.
Large (> 1 mm) papillae in VKC occur predominantly at the upper tarsus. Papillae that may attain a size of 7–8 mm are known as cobblestone papillae (Fig. 2). Papillae size correlate positively with the persistence or worsening of symptoms over long-term follow-up (Bonini et al. 2000). These papillae become quite swollen during the active stage but persist even during the quiescent stage. Limbal papillae tend to be gelatinous and confluent (Fig. 3).
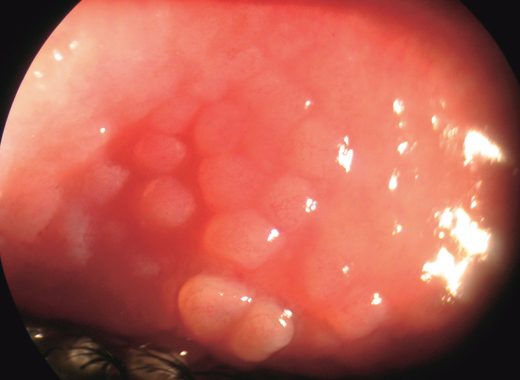
Cobble stone papillae.
Limbal papillae.
Bonini et al. (2000) graded the papillae on the upper tarsal conjunctiva or at the corneoscleral limbus as follows:
- 1
Grade 0: no papillary reaction.
- 2
Grade 1+: few papillae, 0.2 mm widespread over the tarsal conjunctiva or around the limbus.
- 3
Grade 2+: papillae of 0.3–1 mm over the tarsal conjunctiva or at the limbus.
- 4
Grade 3+: papillae of 1–3 mm all over the tarsal conjunctiva or for 360° around the limbus.
- 5
Grade 4+: papillae of more than 3 mm over the tarsal conjunctiva or gelatinous appearance at the limbus covering the peripheral cornea.
Based on the predominant involvement of either tarsal or limbal conjunctiva, VKC can be divided into palpebral, bulbar or mixed type with limbal forms being prevalent in non-White patients (Verin et al. 1999).
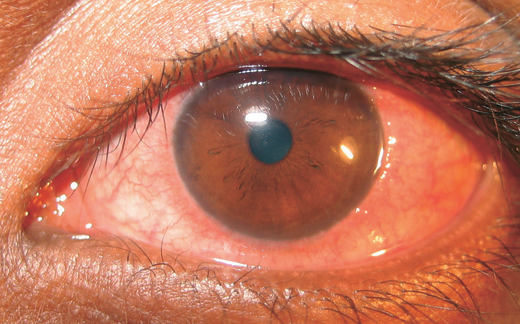
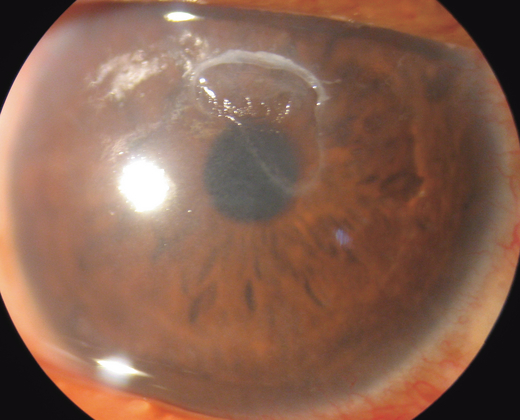
Photophobia, pain and foreign body sensation are caused by involvement of the cornea. Corneal changes include punctate epithelial keratitis, epithelial macro-erosions, shield ulcer, plaque formation and late corneal vascularization (Allansmith & Ross 1988; Buckley 1988). Coalescence of punctate epithelial keratitis areas leads to frank corneal epithelial erosion, leaving Bowman’s membrane intact. If untreated, a plaque containing fibrin and mucus deposits over the epithelial defect (Rahi et al. 1985). Epithelial healing is then impaired, and new vessel growth is encouraged. The oval-shaped epithelial defects, known as shield ulcers (Fig. 4), usually have their lower border in the upper half of the visual axis. Healed shield ulcers may leave a subepithelial ring-like scar (Fig. 5). Corneal ulcer is reported to occur in 3–11% of patients. Corneal changes cause permanent reduction in visual acuity in 6% of patients suffering from VKC (Neumann et al. 1959; Cameron 1995a; Tabbara 1999). Pseudogerontoxon, which resembles arcus senilis, is a waxing and waning grey-white lipid deposition in the superficial stroma of the peripheral cornea.
Shield ulcer.
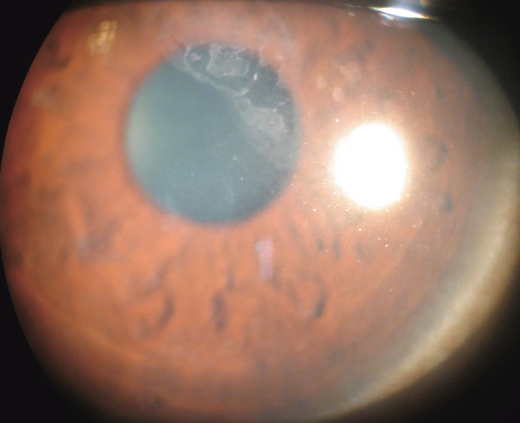
Ring-shaped corneal opacity after healed shield ulcer.
Signs of VKC are confined mostly to the conjunctiva and cornea. The skin of the lid and lid margin are relatively uninvolved. The conjunctiva of the fornices does not usually show foreshortening and symblepharon formation. Iritis is not reported to occur in VKC. Ocular complications of VKC have been reported to include steroid-induced cataract and glaucoma, corneal scarring, microbial keratitis and limbal tissue hyperplasia (Sridhar et al. 2003). Amblyopia seen among VKC may be caused by corneal opacity, irregular astigmatism and keratoconus. Dry eye syndrome, reported in patients suffering from VKC, may be caused by unsupervised use of topical corticosteroids (Tabbara 1999).
No precise diagnostic criteria have been established for this disease. Hyperaemia, itching, photophobia, tearing and mucus discharge are typical symptoms of VKC. Large papilla on the upper tarsal conjunctiva and corneoscleral junction are hallmarks of VKC. Diagnosis is based on typical clinical signs and symptoms, thus many mild or atypical cases may escape diagnosis. The lack of standardized diagnostic criteria and lack of common language among physicians regarding the severity of VKC renders this disease more difficult to diagnose and treat. Bonini et al. (2007) proposed a clinical grading according to clinical phases of VKC to help physicians use a common language in the diagnosis and management of VKC and to allow a more homogenous selection of patients for clinical trials. Despite mounting data suggesting the role of both IgE and non-IgE mediated immune responses in the pathogenesis of VKC, no clinical or laboratory test has evolved to support the diagnosis in atypical cases or predict the course of this disease (Bonini et al. 2000).
Pathophysiology
Numerous cytological, immunohistological and molecular biological studies of allergic inflammation have facilitated our understanding of the pathophysiology of VKC. The ability to measure cytokines in tears, along with in-vitro analysis of the individual or combined effects of these cytokines on conjunctival mast cells, epithelial cells and fibroblasts has facilitated our understanding of specific processes contributing to the pathogenesis of VKC. The clear abundance of Th2 cytokines, upregulated expression of their receptors and conspicuous paucity of T helper cell type 1 (Th1) cytokines in tear and serum of VKC patients confirm the crucial role played by these factors in the onset and perpetuation of the chronic allergic inflammation observed in VKC. A variety of cells, normally present or infiltrating the ocular surface, are responsible for the profound expression of these cytokines in VKC. The factors stimulating these varied cells to increase their cytokine production are still poorly defined. The immune, nervous and endocrine systems seem to interact with each other in the pathogenesis of VKC (Bonini et al. 2004). Infective factors were thought to contribute to the pathogenesis of VKC but respiratory syncytial virus and chlamydia were not detected in conjunctival biopsies from patients with VKC (Koulikovska et al. 2001). Furthermore, serology for ocular chlamydial disease was also negative (Montan et al. 1999).
Personal or family history of atopy, elevated serum level of total and specific IgE, higher number of mast cells and eosinophils, increased level of mediators and favourable response to anti-allergic therapy – the features of allergic diseases – are also observed in VKC (Allansmith 1982; Bielory & Frohman 1992; Abelson & Schaefer 1993).
Conventionally, VKC was considered primarily a type 1 hypersensitivity reaction but the IgE-mast-cell-mediated process is not enough to explain the various clinical and histopathological changes associated with VKC (Coombs & Gell 1962; Allansmith 1982; Johansson et al. 2001). Negative response to skin tests or radioallergosorbent test for common allergens in almost 50% of patients, along with the presence of a multitude of cells and mediators in the conjunctiva, tears and serum of VKC patients, suggest a more complex pathophysiology than a mere type 1 hypersensitivity reaction (Bonini et al. 2000). The prevalence of IgE sensitization was significantly lower in bulbar than in tarsal and mixed VKC. Higher serum eosinophil cationic protein (s-ECP), total serum IgE (s-total IgE) and peripheral blood eosinophil counts have been reported in IgE-sensitized than in non-IgE-sensitized VKC patients (Pucci et al. 2003). Interestingly, Montan et al. (2002) found that the amount of mRNA encoding Th2-type cytokines and inflammatory cell markers in VKC remain the same irrespective of IgE sensitization.
Mediators in VKC
The plethora of mediators and cytokines in VKC compared to controls, seasonal allergic conjunctivitis and giant papillary conjunctivitis provides a new perspective on the complex inflammatory processes occurring on the ocular surface in this chronic disease (Cook 2004).
Cytokines
Cytokines are small secreted proteins that mediate and regulate immunity and inflammation. Unlike hormones, these are not stored as preformed molecules but must be produced, de novo, in response to a stimulus. Different cell types may secrete the same cytokine or a single cytokine may act on several different cell types. Similar functions can be stimulated by different cytokines. ILs are the cytokines that are made by leukocytes and act on other leukocytes.
Activated helper T cells (CD4), mast cells and eosinophils are the main cytokine-producing cell types infiltrating the conjunctiva during chronic allergic eye diseases. Two distinct subtypes of helper T cells produce different cytokines. T cells isolated from conjunctiva of VKC patients and expanded into cell lines showed a Th2-like cytokine profile (Calder et al. 1999).
Th2 cytokines, i.e. IL-4 and -5, were high among VKC patients (Metz et al. 1997; Calder et al. 1999; Leonardi et al. 1999a). Increased expression of mRNA encoding Th2-type ILs was observed in allergic tissue from VKC (Metz et al. 1997; Mori et al. 2002). The serum levels of IL-4 and tear levels of IL-4,-5 were higher in patients with VKC compared to controls. Interestingly, IL-2, interferon (IFN)-gamma and tumour necrosis factor (TNF)-β, the major cytokines secreted by Th1, were not increased in VKC (Leonardi 2002a; Mori et al. 2002). These findings confirm that VKC has a mainly Th2 profile (Uchio et al.2000; Fujishima et al. 2002).
Chemokines
Chemokines, a short term for chemotactic cytokine (CC), are potent activators and chemoattractants. Chemokines are produced not only by inflammatory cells but also by stimulated epithelial cells, fibroblasts and vascular endothelial cells in the conjunctiva. Chemokines are involved in normal trafficking of leukocytes and recruitment during inflammation but their role is not restricted to cell attraction. These multipotent cytokines localize and enhance inflammation by inducing chemotaxis and cell activation of different types of inflammatory cells present at sites of inflammation. Chemokines bind to transmembrane G-protein coupled receptors (CCRs), which then signal the cells via secondary messengers to alter its behaviour.
Chemokines are grouped into the CXC, CC, C and CX3C subfamilies (Murphy et al. 2000). The CC chemokines – monocyte chemotactic protein (MCP), regulated upon activation, normal T cells expressed and secreted (RANTES), macrophage inhibitory protein (MIP), thymus and activation-regulated chemokine (TARC) and eotaxin – act on eosinophils, basophils, monocytes and lymphocytes, suggesting their important role in allergic eye diseases (Abu El-Asrar et al. 2000).
High levels of eotaxin were found in mucus from VKC patients. Eotaxin levels correlate significantly with the percentage of eosinophils in tears and may be responsible for the eosinophil recruitment in VKC (Leonardi et al. 2003a). Eotaxin, along with MCP and RANTES, were highly expressed in limbal tissues (primarily by macrophages) and were responsible for the massive eosinophil infiltration in this tissue (Abu El-Asrar et al. 2000).
IL-8 and the CXC chemokine, monokine induced by interferon gamma (Mig), seem to play an important role in the pathogenesis of VKC. The chemokine IL-8 actively secreted by macrophages and epithelial cells in VKC is a chemoattractant as well as an activator of polymorphonuclear cells. It plays a crucial role in inflammatory cell migration. More polymorphonuclear cells and eosinophils have been correlated with increased IL-8 concentrations (Miyoshi 2001).
Chemokine receptor (CXCR)-3 is greatly upregulated and expressed abundantly on T lymphocytes in the conjunctiva of patients with active VKC. Over-expression of this receptor and chemokine Mig may play an important role in the regulation of lymphocyte recruitment within the conjunctiva of VKC patients (Abu El-Asrar et al. 2001a, 2003).
Histamine
Histamine, an important inflammatory mediator in allergic eye disease, is released by activated mast cells and basophils. Tear concentration of histamine was higher in VKC patients compared to normal volunteers and other inflammatory eye diseases (Abelson et al. 1980). Persistent elevation of histamine levels in VKC tears is probably caused by its reduced inactivation by histaminase and increased production by specific or non-specific activation of mast cells and basophils (Abelson et al. 1995). Patients affected with VKC demonstrate a non-specific conjunctival hyper-reactivity to histamine (Bonini et al. 1992a).
Skin test reactivity to histamine also showed a greater wheal and flare response in VKC patients, further confirming a hypersensitivity to histamine. In vitro, histamine has a stimulating effect on conjunctival fibroblasts, increasing the production of procollagen I (Leonardi et al. 1999b). In a study by Weimer et al. (1998), histamine was shown to stimulate the conjunctival epithelial cells H1 receptors to produce cytokines. Thus, histamine not only causes pruritus and hyperaemia but it also seems to participate in allergic inflammation and tissue remodelling by altering the behaviour of the conjunctival epithelium and fibroblasts.
Metalloproteinases
Metalloproteinases (MMPs) are extracellular endopeptidases that selectively degrade components of the extracellular matrix. Inflammatory cells, particularly eosinophils, and structural cells like epithelial cells and conjunctival fibroblasts are the probable cellular source of these enzymes.
Different cytokines act on fibroblasts to up- or downregulate the production of collagen and modify the equilibrium between matrix MMP-1 and its inhibitor, tissue inhibitor of MMP (TIMP)-1 (Leonardi et al. 2003b). Tear levels of pro-MMP-1 and pro-MMP-9 were significantly increased in patients with VKC compared to control subjects. MMP-9 activity correlated significantly with corneal involvement and giant papillae formation (Leonardi et al. 2003c).
The increased production and activation of MMPs or imbalance between MMPs and their natural tissue inhibitors (TIMPs) are all probably involved in the pathogenesis of conjunctival inflammation, remodelling and corneal changes in VKC.
Growth factors
Several growth factors, such as epidermal growth factor, fibroblast growth factor and transforming growth factor beta-1 (TGFβ-1), were increased in VKC. These factors induce fibroblast growth and procollagen production (Leonardi et al. 1998). Recently, receptors for nerve growth factor were detected in the conjunctiva of patients with active VKC. High plasma levels of nerve growth factor detected in VKC correlate with the number of mast cells in conjunctival tissue, suggesting that neural factors may have a role in the pathogenesis of VKC (Lambiase et al. 1995). Interestingly, substance P – a neuropeptide with well-known activity on immune cells – was detected in tear and plasma of VKC patients (Fujishima et al. 1997; Lambiase et al. 1997).
Cells in VKC
Mast cells, T cells, eosinophils and macrophages are seen in increased numbers among VKC patients.
Mast cells
The mast cells are the key cellular component and play a pivotal role in initiating the inflammatory cascade in allergic eye disease. In histopathology, mast cells are the constant feature of conjunctival tissue from VKC. The greatly increased number of mast cells found in tissue samples from tarsal giant papillae suggests an active role for these cells in the abnormal connective tissue metabolism observed in VKC.
These cells express Fc [epsilon] RI on their cell surface, which enables them to bind IgE (Church & Levi-Schaffer 1997). The cross-linkage of this IgE by specific allergens results in the release of pro-inflammatory mediators, including histamine, proteases, prostaglandin D2 and leukotriene C4, into the local extracellular environment. These mediators are responsible for causing ocular itching, hyperaemia, lacrimation and chemosis in allergic conjunctivitis (McGill et al. 1998; Church & McGill 2002). Among mast-cell-derived mediators, histamine is a potent and abundant vasoactive agent. Histamine exerts its biological effects by interacting with four G-protein coupled receptors, classified as H1–H4. Vasodilatation, chemosis and itching of eye are caused by histamine interaction with H1 receptors.
Based on their neutral protease content, the mast cells are divided into two subtypes: the mast-cell phenotype containing only tryptase in their granule, known as MCT, and that containing both tryptase and chymase, called MCTC (Irani et al. 1986). In normal patients, approximately 80% of conjunctival mast cells are of MCTC type. The number of MCT with a primary role in host defence and allergic diseases were increased in conjunctiva from VKC (Miyazaki et al. 2004).
It has been shown that mast cells in conjunctiva can synthesize IL-4 (Anderson et al. 2001). Mast-cell cytokines are responsible for the initiation of allergic inflammation, resulting in eosinophil infiltration associated with vernal conjunctivitis (Church & Levi-Schaffer 1997). IL-4 plays a key role in allergic inflammation by promoting T cell growth, induction of IgE production from B cells, upregulation of adhesion molecules and regulation of Th2 subset differentiation, which is essential for the allergic reaction. Furthermore, IL-4 is reported to induce eotaxin production in keratocytes, which may promote eosinophil recruitment to corneal ulcer (Fukagawa et al. 2000). Tryptase and chymase, indicators of mast-cell activation, are increased in tears and may serve as sensitive markers for determining the severity of VKC (Tabbara 2001; Ebihara et al. 2004).
Eosinophils
Eosinophils are the constant feature of lacrimal and conjunctival cytology of VKC. Approximately 50–90% of cells in the tears during the active phase of VKC are eosinophils (Leonardi 2002a). Eosinophils, along with mast cells, are the main effector cells in ocular inflammation in VKC. Numbers of eosinophils are increased significantly in the tears, peripheral circulation and conjunctival tissue from VKC patients. The eosinophil infiltration of the conjunctiva is not affected by the corneal involvement. The presence of degranulated eosinophils as well as ECP and eosinophil major basic protein (MBP) – the toxic enzymes liberated by these cells – in the tears, conjunctiva and periphery of corneal ulcers in VKC patients indicate the significant role played by eosinophils in the aetiopathogenesis of VKC (Trocme et al. 1989, 1993; Leonardi et al. 1995; Montan & van Hage-Hamsten 1996). Eotaxins are potent chemoattractants, which recruit and activate eosinophils in VKC (Leonardi et al. 2003a). IL-5 induces eosinophil differentiation, recruitment, activation and survival (Takatsu 1992).
Recently, it has been observed that eosinophils can produce cytokines, particularly in allergic disease, although the disease-specific cytokine spectrum of tissue eosinophils is unknown. Activated eosinophils release cytokines, chemokines, leukotrienes and epitheliotoxic proteins such as MBP, ECP, eosinophil peroxidase (EPO) and eosinophil protein X/neurotoxin (EPX) (Tomassini et al. 1994; Leonardi et al. 1995; Leonardi 2002a).
The tear and serum levels of ECP and EPX are higher in VKC patients in comparison to normal subjects (Bonini et al. 1992a; Leonardi et al. 1995; Montan & van Hage-Hamsten 1996). ECP tear levels correlate positively with clinical signs and symptoms and were reduced when treatment with dexamethasone or cyclosporine was instituted (Leonardi et al. 1995). Tear levels of ECP have been used to evaluate the efficacy of drugs in the treatment of VKC (Leonardi et al. 1997). Higher serum ECP levels do not correlate with severity of ocular symptoms, but the total score of giant papillae correlated strongly with serum ECP and peripheral blood eosinophil counts (Leonardi et al. 2000a; Pucci et al. 2003).
Activated eosinophils, with their mediators and adhesion molecules, play an important role in eliciting ocular surface inflammation and corneal epithelium damage (Trocme et al. 1993; Trocme & Aldave 1994; Bacon et al. 1998). Eosinophil MBP deposition, found in corneal ulcers of VKC patients, suggests the direct deleterious effects of eosinophil proteins on corneal epithelium (Trocme et al. 1993). Gelatinase B is over-expressed by eosinophils in conjunctiva specimen from VKC patients (Abu El-Asrar et al. 2001b). This enzyme may function with other eosinophilic proteins to damage corneal epithelium.
T cells
Numbers of T lymphocytes increase in the conjunctiva of patients with VKC. The activation of these lymphocytes appears to play a vital role in the pathogenesis of chronic allergic inflammation seen in VKC.
T cell clones derived from VKC tissue are mainly of Th2 type (Maggi et al. 1991). Cytokine flow cytometry has shown that 67% of VKC patients have Th2 cells in tears, while Th1 cells are seen in the tears of only 8% (Leonardi et al. 1999a). The predominance of Th2-like cells in tears and conjunctival biopsy suggests a local Th2 response in VKC. Th2 lymphocytes, by virtue of their cytokine profile, are responsible for increased production of IgE, recruitment and activation of mast cells and eosinophils (Umetsu & De Kruyff 1997; Bielory et al. 2002a).
These T cells actively interact with other inflammatory cells, such as macrophages. Co-stimulatory molecule CD86 – critical for successful antigen presentation and the development of Th2 immune response – is prominently expressed by Langerhan’s cells in conjunctiva specimen from VKC (Abu El-Asrar 2001c). Thus, antigen-presenting cells provide an important mechanism for Th2 cell activation.
The mechanisms of T cell recruitment within the conjunctiva remain poorly defined, despite mounting evidence suggestive of T cell involvement in VKC. Trafficking of activated T cells into inflammatory sites is a tightly controlled process directed by multiple molecules (Butcher & Picker 1996; Rossi & Zlotnik 2000). Intercellular adhesion molecule-1 (ICAM-1), CC chemokines, macrophage-derived chemokine, E-selectin and human leukocyte antigen DR (HLA-DR), over-expressed in VKC and acting concomitantly, may be responsible for the recruitment and activation of helper T cells in VKC.
B cells
B lymphocytes expressing the ligands CD23, 21 and 40 in conjunctiva specimen from VKC may be a precursor of IgE producing B cells (Abu El-Asrar et al. 2001d).
Natural killer cells
Lambiase et al. (2007) reported the increased natural killer cells in the conjunctiva of patients with VKC, suggesting a potential role of these cells and innate immunity in VKC.
Epithelial cells
It has been shown that conjunctival epithelial cells not only act as a mechanical barrier, but also participate in the regulation of allergic inflammation through expressing surface antigens such as adhesion/effector molecules (ICAM-1, vascular cell adhesion molecule-1 and HLA-DR) and releasing many cytokines (eotaxin, IL-8, IL-6, RANTES). Histamine, released from the conjunctival mast cells, might stimulate the synthesis of IL-6 and IL-8 by conjunctival epithelial cells and amplify the allergic response (Irkec & Bozkurt 2003).
ICAM-1, HLA-DR, IL-3 and GM-CSF are not expressed in normal conjunctival epithelium but these antigens were induced on conjunctival epithelial cells in VKC. RANTES, present in normal conjunctival epithelial cells, was upregulated in VKC. ICAM-1 may allow epithelial cells to recruit, retain and locally concentrate leukocytes. The upregulated epithelial cytokines are known to promote eosinophilic inflammation. Expression of HLA-DR may play an important role in antigen presentation by conjunctival epithelial cells (Hingorani et al. 1998b).
Fibroblast
It has been shown that corneal and conjunctival fibroblasts not only maintain tissue structure but also contribute to the induction and amplification of ocular allergic inflammation as well as tissue remodelling. TGFβ-1, IL-1 and Th2 cytokines from allergic inflammatory cells induce vascular endothelial growth factor (VEGF) production in conjunctival fibroblasts, which may play a crucial role in neovascularization and formation of giant papillae (Asano-Kato et al. 2005).
Corneal fibroblasts express a high-affinity receptor complex for IL-4 and IL-13. Stimulation of corneal fibroblasts with IL-4 or IL-13 induces expression of eotaxin and vascular cell adhesion molecule-1 (VCAM-1), which together mediate eosinophil infiltration into the cornea (Leonardi et al. 2003a; Fukuda 2005). A potent chemoattractant for Th2 cells, thymus-activation-regulated chemokine (TARC), is expressed by corneal fibroblast upon stimulation by IL-4 and IL-13.
In vitro, corneal fibroblasts expressed ICAM-1 and VCAM-1 when activated with IL-4 and TNF-α. Eosinophils can adhere to the activated fibroblasts and induce subsequent fibroblast damage through these adhesion molecules. Eosinophil adhesion to fibroblasts may contribute to the pathogenesis of severe persistent allergic corneal ulcers (Okada et al. 2005).
Fibroblasts can modulate the functions of mast cells and eosinophils through the membrane form of stem cell factor and GM-CSF, respectively. On the other hand, fibroblasts can be affected by inflammatory mediators derived from mast cells and eosinophils, such as TGFβ and nerve growth factor and by the Th2 cytokines, IL-4 and IL-13 (Solomon et al. 2003).
Histopathology and immunohistochemistry
Histopathological studies of conjunctival tissue from VKC patients show a prominent inflammatory cellular infiltration in the epithelium and substantia propria and post-inflammatory tissue remodelling. Tissue remodelling is more marked in tarsal than in bulbar conjunctiva.
Tissue inflammation
The histopathology of VKC is characterized by infiltration of the conjunctiva by eosinophils, basophils, mast cells, Th2 cells, monocyte/macrophages, dendritic cells, plasma cells and B lymphocytes, frequently organized as small lymphoid follicles without a germinative centre (Abu El-Asrar et al. 2001d; Leonardi 2002a). The cellular infiltration of both the stroma and the epithelium was consistent with chronic inflammation, particularly in the biopsy specimens taken from the limbus.
Eosinophils are characteristic constituents of the cellular infiltrate in all stages of the disease. Ultra-thin sections of conjunctiva of patients suffering from VKC showed higher numbers of eosinophils in epithelium and subepithelium of conjunctiva (Hingorani et al. 1998a). In the tarsal conjunctiva, the stromal infiltrate consisted of a diffuse T-lymphocyte reaction with clustering of B-lymphocytes, mast cells, IgE plasma cells and eosinophils. Clustering of B lymphocyte was noted more in patients from tropical areas than in those from temperate zones. In comparison with control subjects, patients with VKC were more likely to show squamous metaplasia of the tarsal conjunctiva (Tuft et al. 1998).
Tissue remodelling
The overgrowth of the conjunctival connective tissue, with the formation of large and sessile papillae on the upper tarsal conjunctiva, is one of the most notable findings in VKC. Conjunctival thickening, subepithelial fibrosis, mucus metaplasia, neovascularization and scarring are typical of chronic VKC. Epithelial changes, connective tissue deposition, oedema, inflammatory cell infiltration and glandular hypertrophy all contribute in the tissue remodelling observed in VKC (Leonardi 2002a).
On morphometric measurements, the thickness of the entire mucosa and basement membrane and superficial epithelial area increase in VKC tissue compared to normal (Leonardi et al. 2000b). On histological examination, the number of goblet cells increased in giant papillae and epithelial in-growth. Epithelial in-growth in stroma is rich in goblet cells and may have a pseudoglandular appearance. Collagen fibres in tissue samples from tarsal giant papilla were thicker and arranged irregularly, with the total amount significantly increased.
A proliferation of capillaries and neovascular formations provide vascular support to the papillae. Various growth factors, e.g. epidermal growth factor fibroblast growth factor, TGFβ-1 and many ILs were increased in supernatants of VKC tissue culture compared to normal subjects (Leonardi 2002a).
Plasminogen activators, in addition to fibrinolysis, also play a role in chemotaxis and collagen degradation. Higher levels of serine protease urokinase, tissue plasminogen activator and tear plasminogen activity in VKC patients suggest that increased expression of fibrinolytic system components and imbalance between plasminogen activators and plasminogen activator inhibitors may contribute to tissue remodelling in VKC (Leonardi et al. 2005).
Treatment
Preventive measures and patient education
Compliance with instructions is better with a well-informed patient and outcome of treatment is gratifying. Education of patients and their parents about the chronic, recurrent and ultimately resolving nature of VKC is a very important aspect of management. Because exposure to non-specific stimuli causes frequent conjunctival redness among VKC patients, avoidance of triggering factors like sun, wind and salt water are helpful but not enough to control the symptoms. Contact with commonly known allergens like plants and flowers should be avoided. Use of sunglasses or any shading measures are helpful and should be advised. Application of cold compresses and use of artificial tears have been shown to be effective in the relief of symptoms by direct removal and dilution of allergen from the ocular surface (Bielory 2002b). Cold compresses provide symptomatic relief, especially from ocular pruritus. Frequent hand, face and hair washing – especially before going to bed –, may be helpful (Leonardi 2002a).
Pharmacological therapy
The variety of currently available drugs to treat VKC include anti-histamines, mast-cell stabilizers, dual acting agents, corticosteroids and immunomodulators but none is enough to treat all aspects of multifaceted pathophysiology of VKC. Most drugs used are merely palliative and do not eliminate the complex immune response initiating and perpetuating the allergic ocular inflammation, so there is recurrence of disease when the therapy is discontinued. Because there are few randomized control trials, the selection of a drug from the many available options is mostly based on the personal experience and preference of the treating physician. A meta-analysis of randomized control trials will be helpful in formulating the guidelines for the treatment of VKC.
Judicious and scrupulous use of medication cannot be overemphasized because drug treatment is prolonged and frequent. In some patients, medication needs to be used throughout the year for satisfactory control of symptoms.
Vasoconstrictor and non-specific histamines receptor blocker
Vasoconstrictor and non-specific anti-histamine combination eyedrops have long been available and have withstood the test of time. They contain vasoconstrictors like naphazoline or tetrahydrozoline and anti-histamines like pyrilamine or pheniramine. These drops are safe and effective, at least temporarily. Because of their over-the-counter availability, they are tried by many patients during the early stage of the disease as first-line treatment. By virtue of their vasoconstrictor and non-specific anti-histamine constituents, they relieve itching and reduce redness. Burning or stinging on installation and rebound hyperaemia are common side-effects with these drugs.
Mast-cell stabilizers
Mast-cell degranulation is a central event in VKC, so the treatment has been concentrated on preventing the degranulation or antagonizing the effects of the primary mediator (histamine) released by mast cells. Mediators released by mast cells are responsible for many symptoms and signs associated with VKC. Drug modulation of mast-cell activity alleviate the acute symptoms of active disease and also reduce the cytokine stimulus for the development of chronic allergic inflammation (Church & McGill 2002).
The drugs of this group stabilize mast cells and prevent degranulation. The efficacy of sodium cromoglycate (qid), lodoxamide (qid), nedocromil (bid) and pemirolast (qid) in the control of symptoms and prevention of exacerbation has been shown by many studies (Tabbara & Arafat 1977; Bonini et al. 1992b; Caldwell et al. 1992). These should be used as a first line of defence at the onset of the allergic season and should be used continuously throughout the season.
Cromolyn (4%), the prototype mast-cell stabilizer, has been prescribed by ophthalmologists for the treatment of ocular allergy for the last 25 years. It can be used for long periods without side-effects but takes several days to reach full effect (Sorkin & Ward 1986). Sodium cromoglycate may have a synergistic effect in combination with a corticosteroid for the treatment of VKC (Dahan & Appel 1983).
Lodoxamide 0.1% has been shown to deliver greater and earlier relief in patients with VKC (Fahy G et al. 1988). Lodoxamide was found to control the symptoms and signs of VKC better compared to 4% cromolyn sodium and livocabastine (Leonardi et al. 1997; Avunduk et al. 2000; Verin et al. 2001). Better efficacy of lodoxamide was linked to significantly decreased CD4 cells and inflammatory cells – especially eosinophils in conjunctiva.
Nedocromil (2%), with stabilizing effects on mast cells, also inhibits the chemotaxis, activation and mediator release from other inflammatory cells. Nedocromil sodium and lodoxamide were shown to be superior to sodium cromoglycate in the treatment of VKC but a meta-analysis of the efficacy and effectiveness of topical mast-cell stabilizers in the treatment of seasonal allergic conjunctivitis found insufficient evidence to recommend one drug over another (Verin et al. 1999; Owen et al. 2004). Pemirolast, a pyridopyrimidine compound with mast-cell stabilizing properties, is effective in the treatment of allergic conjunctivitis.
Systemic anti-histamines
Oral anti-histamines are a good choice when allergy involves the eyes, nose or pharynx simultaneously. When allergic complaints are limited to eyes, focused therapy with topical anti-histamines is efficacious and free of untoward effects related to oral anti-histamines. Topical anti-histamines provide faster and superior relief than systemic anti-histamines and possess longer duration of action than topical vasoconstrictors, pure mast-cell stabilizers, non-steroidal anti-inflammatory drugs and corticosteroids, the drugs commonly used in the treatment of ocular allergy.
H1 receptor blocker
Topical selective H1 blocker, emedastine and levocabasitne, are better than vasoconstrictor/non-specific anti-histamine combination eyedrops in controlling the signs and symptoms of VKC. Levocabastine (0.05%) is a potent topical anti-histamine with rapid and prolonged selective anti-H1 receptor activity. Many studies have shown it to be effective in treatment of seasonal allergic conjunctivitis when compared to placebo. Emedastine (0.05%) is a relatively selective H1 receptor blocker with no apparent effects on adrenergic, dopaminergic or serotonin receptors. The H1 receptor antagonist emedastine inhibits cytokine production by conjunctival epithelial cells (Weimer et al. 1998).
The enhanced clinical efficacy of emedastine, levocabastine and (recently) olopatadine over first-generation anti-histamines antazoline and pheniramine may be the result of the inhibitory effects of new-generation anti-histamines on the pro-inflammatory cytokines produced by conjunctival epithelial cells. The anti-inflammatory effect seen with pure anti-histamines like levocabastine and emedastine is attributed to the blocking of histamine receptors, thus downregulating the expression of ICAM-1 and limiting chemotaxis of inflammatory cells (Bielory et al. 2005).
H1 receptor blocker and mast-cell stabilizer (dual action drugs)
Recently, a new generation of drugs such as olopatadine, epinastine, ketotifen and azelastine has shown dual activity of mast-cell stability and H1 receptor antagonism. Fast H1 receptor blocking action, along with mast-cell stabilization, makes them suitable for twice-daily dosing. The action of these drugs is not limited to mast-cell stabilization and H1 receptor antagonism: they also exert anti-inflammatory effects through several different mechanisms.
Olopatadine (0.1%) is a selective H1 antagonist with mast-cell-stabilizing properties. Olopatadine, in addition to stabilizing mast cells, affects the release of TNF-α and various cytokines from conjunctival epithelial cells, thus controlling the allergic inflammation more effectively than the other topical anti-histamine formulations (McGill 2004). It decreases the mucus discharge in VKC by reducing the goblet cell density in the conjunctiva (Corum et al. 2005).
Ketotifen (0.025%) is a selective non-competitive blocker of the H1 receptor. Besides mast-cell stabilization and H1 receptor antagonism, it prevents eosinophil accumulation. Ketotifen has been shown to inhibit the release of inflammatory mediators from basophils and eosinophils, chemotaxis, leukotriene activity and platelet activation, and also to decrease vascular permeability. Epinastine (0.05%) is an H1 receptor antagonist with mast-cell-stabilizing and anti-inflammatory activity. Azelastine (0.05%) is a selective H1 receptor antagonist that inhibits histamine release. It downregulates ICAM expression, reduces eosinophil chemotaxis and inhibits platelet-activating factors.
Non-steroidal anti-inflammatory
Topical formulations of ketorolac and diclofenac have been shown to diminish ocular pruritus and conjunctival hyperaemia associated with allergic conjunctivitis. Prostaglandin E2 and I2 has been shown to be pruritogenic. In a prospective, open study, preservative-free diclofenac (0.1%) eyedrops reduced the symptoms of VKC through the inhibition of prostaglandin production in 40% of patients. Although the conjunctival hyperaemia reduced significantly, the papillary size and corneal lesions remained unchanged (D’Angelo et al. 2003).
In a prospective, randomized cross-over study, preservative-free ketorolac tromethamine (0.5%) reduced the signs and symptoms of VKC more rapidly than topical cyclosporine (0.5%) (Kosrirukvongs & Luengchaichawange 2004). Ketorolac may be a good alternative to topical steroid because it reduces itching by inhibiting the synthesis of prostaglandins (Sharma 1997).
Corticosteroids
Topical corticosteroids are one of the most effective drugs to control the signs and symptoms of VKC. Because of complications associated with their prolonged use, these should not be prescribed as first-line treatment. Prolonged application of corticosteroids may cause steroid-induced cataract, glaucoma and increase susceptibility to viral and fungal infections. Recently, modified steroids such as loteprednol etabonate and rimexolone were developed. In comparison to other steroids, loteprednol has a superior safety profile, which has been attributed to its ‘soft drug’ characteristics.
Loteprednol is highly effective in the acute and prophylactic treatment of allergic conjunctivitis. In a retrospective study, loteprednol etabonate was shown to be safe and effective when used for 12 months or more for the treatment of seasonal or perennial allergic conjunctivitis (Ilyas et al. 2004). Rimexolone is a derivative of prednisolone and is inactivated quickly in the anterior chamber.
Fluorometholone is a soft corticosteroid and is effective in controlling the signs and symptoms of VKC. Desonide phosphate has been shown to be as effective as fluorometholone in the treatment of allergic conjunctivitis (Leonardi et al. 2002b).
Supratarsal injection of corticosteroids can be used to treat VKC refractory to conventional treatment (Holsclaw et al. 1996). Supratarsal injection of dexamethasone sodium succinate, triamcinolone acetonide and hydrocortisone sodium succinate is effective in the temporary suppression of inflammation associated with VKC (Saini et al. 1999; Singh et al. 2002; Lisanework 2003). The resolution of the limbal form of the disease was more dramatic than the palpebral form (Lisanework 2003). Although corticosteroids are the most efficacious drugs, steroid-resistant forms of VKC are not unusual and may necessitate an alternative therapy.
Immunomodulators
Cyclosporine, a fungal metabolite, decreases the signs and symptoms of VKC. BenEzra et al. (1986) used cyclosporine 2% eyedrops in oil solution to treat severe VKC almost two decades ago. They reported rapid relief in subjective symptoms in 86% of VKC patients treated by topical cyclosporine. However, symptoms recurred and required additional therapy after discontinuation of cyclosporine eyedrops in most patients (BenEzra et al. 1988). Cyclosporine is lipophilic so it must be dissolved in an alcohol–oil base, which causes ocular irritation. Cyclosporine (0.5–2%) emulsion in olive or castor oil, instilled four times daily, was shown to be effective in the treatment of VKC. Clinical improvement in VKC after cyclosporine treatment may result from its immunomodulating effect on components of cell-mediated and humoral immune response. (Abu El-Asrar et al. 1996). Cyclosporine blocks Th2 lymphocyte proliferation and IL-2 production. Furthermore, it inhibits histamine release through a reduction in IL-5 production (BenEzra et al. 1988; Secchi et al. 1990). Accelerated apoptosis induced by cyclosporine in fibroblast cultures obtained from a patient with VKC suggests a potential role for this drug in hyperproliferative conjunctival disorders like VKC (Leonardi et al. 2001).
In double-blind, placebo-controlled trials, cyclosporine (2%) eyedrops were effective and safe in the treatment of severe VKC (Pucci et al. 2002; Kilic & Gurler 2006). Most of the therapeutic effect was achieved 2 weeks after commencing the treatment and was maintained during the next 3 months by continuous medication (Pucci et al. 2002). In a prospective study of 10 cases with severe VKC, topical cyclosporine (2%) significantly reduced the clinical signs and symptoms score. The reduction of CD4 and CD28 cell populations on the conjunctival surface can be responsible, at least partly, for clinical improvement in VKC after treatment with cyclosporine (Avunduk et al. 2001). Cyclosporine 1% was shown to control the symptoms of VKC, although further control trials are required to find the optimum concentration of cyclosporine needed to treat VKC (Spadavecchia et al. 2006).
Topical cyclosporine helps in the healing of vernal shield ulcers but recurrences may occur at lower concentrations, which can be treated by increasing the concentration (Cetinkaya et al. 2004). Off-label use of commercial preparation of cyclosporine (0.05%) eyedrops, recommended for the treatment of dry eye, decreased the symptoms and signs of VKC (Ozcan et al. 2007). Topical corticosteroids and artificial tears have been shown to act synergistically with cyclosporine 0.05% eyedrops and help in the re-epithelialization of corticosteroid-resistant vernal shield ulcers (Kumar 2008). Whether this commercially available formulation of cyclosporine is effective in the treatment of VKC needs to be tested in controlled trials.
Anti-metabolites
Mitomycin-C is an inhibitor of fibroblast proliferation. Mitomycin-C (0.01%) eyedrops were shown to decrease the mucous discharge, conjunctival hyperaemia and limbal oedema in VKC patients refractory to topical steroids and mast-cell stabilizers (Akpek et al. 2000; Jain & Sukhija 2006).
Future of VKC pharmacological therapy
Despite the development of newer drugs in the last decade, the statement of Professor Lightman –‘at present however, the current situation for those with severe VKC remains a disturbing dependence upon topical steroids, with all the attendant risks’, emphasizing the need for more selective drugs for better and long-lasting control of VKC – is still appropriate (Hingorani & Lightman 1995). Anti-chemokine receptor antibodies inhibit eosinophil chemotaxis. Inhibition of CC chemokine receptor-3 may be a treatment for corneal ulceration in patients with VKC (Fukagawa et al. 2002). So far, results with topical cyclosporine are very encouraging but because of unavailability of commercial preparations of topical cyclosporine in higher concentrations, its use in VKC is limited. Future developments in the manipulation of eosinophilic products, cytokines and adhesion molecules may also be relevant. High levels of leukotrienes in the tears of patients with VKC and improvements in the signs and symptoms of VKC when given oral montelukast, a leukotriene receptor antagonist, suggest that anti-leukotrienes have therapeutic potential and need further trials (Akman et al. 1998; Lambiase et al. 2003). Macrobiomolecules have been shown to inhibit ocular allergic responses. Anti-flammins, a macrobiomolecule, inhibit phospolipase A2. Topical anti-flammins have been shown to inhibit allergic responses in murine allergic conjunctivitis. Immunostimulatory oligonucleotides (ISS) or CpG motiff, another macrobiomolecules, inhibits ongoing TH2 response in murine allergic conjunctivitis (Tuo & Chan 2003). Probiotics have been shown to improve allergic inflammation. Furthermore, a report by Iovieno et al. (2008) has shown that Lactobacillus acidophilus diluted in saline solution administered as eyedrops improves signs and symptoms in patients with VKC.
Surgical treatment
Surgical excision of giant papillae is recommended if they cause corneal lesions. Excision or cryocoagulation of large papillae helps in the early resolution of corneal epitheliopathy or ulcer, although papillae regrow in most patients. Cryotherapy of giant papillae promotes inflammation and may cause conjunctival scarring. Intraoperative application of 0.02% mitomycin-C (MMC) to the upper palpebral conjunctiva immediately after papillae resection for 2 min reduces the chances of recurrence of papillae significantly. No complications related to MMC use were observed during a follow-up period ranging from 12 to 18 months (Tanaka et al. 2004). Giant papillae can be removed by CO2 laser. The procedure can be repeated if papillae recur (Belfair et al. 2005).
Debridement of ulcer base, surgical removal of plaque or excimer laser phototherapeutic keratectomy helps in early re-epithelialization of vernal shield ulcer refractory to medical treatment. (Cameron et al. 1995b; Solomon et al. 2004; Ozbek et al. 2006). Amniotic membrane implantation leads to complete re-epithelialization of persistent corneal epithelial defects and vernal plaques recalcitrant to conventional medical treatment (Rouher et al. 2004; Pelegrin et al. 2008).
Free autologus conjunctival graft after resection of giant papillae facilitates the re-epithelializaion of non-healing shield ulcer (Nishiwaki-Dantas et al. 2000). Cultivated corneal epithelial cells could be transplanted to treat the severe ocular surface diseases associated with VKC. Vision improves significantly after transplant. Corneal epithelial cell transplants could be beneficial when amniotic membrane transplant is not sufficient to restore the ocular surface (Sangwan et al. 2005).
Acknowledgements
Prof. S. Lightman (Moorefields Eye Hospital), Glen Brice (St George’s University of London), Nitin Gupta (West Suffolk Hospital), Jim Stahl (University of Wisconsin Medical School), Philip Thomas (Joseph Eye Hospital), Vijay Sharma (National University, Singapore), Anil Nambiar (SAAD Hospital) and Karuna Sharma (Mohammad Dossary Hospital).




