Abstract
Background: Fetal infection by human parvovirus B19 is a common cause of fetal anemia, nonimmune hydrops fetalis, and spontaneous abortion and can result in fetal death. Recent improvements in diagnosing parvovirus infections and the availability of intrauterine transfusion have reduced the overall rate of fetal loss after maternal exposure.
Methods: We report two cases of maternal parvovirus infection with classic findings of hydrops fetalis and review various aspects of parvovirus infection with emphasis on the developing management options in pregnancy.
Results and Conclusions: Different management led to different results. In the first case there was normal neonatal and infantile development, and in the second case, the fetus died. With accurate laboratory testing, obstetric sonography, and fetal transfusion, the fetal mortality from parvovirus infection has been reduced considerably, and most pregnancies complicated by maternal parvovirus infection result in healthy outcomes.
Parvovirus B19 was first discovered by Cossart and colleagues in 19751 in the sera of asymptomatic patients being screened for hepatitis B infection. In 1983, Anderson et al2 described it as the probable cause of erythema infectiosum, also known as fifth disease. The first association between parvovirus B19 infection in pregnancy and poor outcome was reported in 1984, when hydropic fetuses were shown to have anti-B19 immunoglobulin M (IgM).3 ,4 Multiple reports have confirmed these associations.5–7 A fetus affected by parvovirus B19 might show sonographic signs of generalized edema, subcutaneous edema, ascites, pleural effusion, pericardial effusion, placental edema, and polyhydramnios. The proposed mechanism is severe anemia, which leads to hypoxia and cardiogenic heart failure. With intrauterine transfusions, several authors have reported improved outcomes in cases of moderate anemia caused by hydrops.8
We report two cases of maternal parvovirus infection with the classic finding of hydrops fetalis. Serologic evidence of parvovirus-specific IgM-antibody positivity confirmed the diagnosis. In one of our cases the fetus underwent intrauterine transfusion with a good outcome; in the other case, the fetus did not undergo transfusion according to the family’s wish, and the fetus died.
Illustrative Cases
Case 1
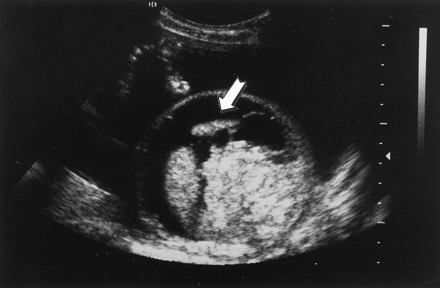
A 25-year-old woman, gravida 4, para 1,1,1,2, was found to have hydrops fetalis on a routine sonographic examination for dating at 25 weeks’ gestation. Ascites and a small pleural effusion were seen in an otherwise normal singleton gestation (Figure 1). No other fetal anomalies were noted, and the amniotic fluid volume was normal. She denied any fever, rash, cold symptoms, and joint pain before and during the pregnancy. Maternal parvovirus B19 infection was confirmed by the finding of positive IgM and immunoglobulin G (IgG) in the serum. A maternal-fetal medicine specialist was consulted.
Sonogram showing hydrops fetalis at 25 weeks’ gestation. Arrow indicate the ascites.
An initial nonstress test at 25 weeks of estimated gestational age showed a baseline fetal heart rate of 130 beats per minute, a nonreactive fetal heart tracing, and several spontaneous decelerations. After discussing management options, at 28 weeks of estimated gestational age, a percutaneous umbilical blood sampling was performed. Testing showed an initial hematocrit of 18% with a mean corpuscular volume of 118 μm3. Fifty milliliters of type O-negative, cytomegalovirus-negative, irradiated blood were transfused. A follow-up hematocrit of the fetus was 45%. The fetal non-stress test was markedly improved after the procedure. The fetal karyotype was normal.
The mother was observed with weekly sonograms and nonstress tests three times weekly. At 30 weeks’ gestation, a sonogram showed resolving hydrops fetalis, and at 31 weeks’ gestation, no sign of fetal hydrops remained (Figure 2). Biweekly fetal and maternal surveillance showed normal progress until the 39th week of gestation, when labor was induced and the patient underwent a normal vaginal delivery. The infant weighed 2,872 g, and Apgar scores were 9 at 1 minute, 10 at 5 minutes. The hemoglobin concentration at birth was 15.5 g/dL and the hematocrit was 46%. The neonatal course was uneventful. Parvovirus titers checked at 2 weeks of life were IgM negative and IgG positive. Newborn growth and development have been normal. At the age of 12 months, the child shows normal growth and neurodevelopment.
Sonogram showing resolving hydrops fetalis after intrauterine transfusion.
Case 2
A 32-year-old woman, gravida 3, para 2,0,0,2, was seen at 17 weeks’ gestation because of a recent maternal parvovirus infection, confirmed by positive IgG and IgM determinations. At 21 weeks’ gestation, minimal fetal ascites were seen on sonographic examination. At 23 weeks’ gestation, the ascites had increased, and a pericardial effusion had developed. Repeated sonograms showed fetal hydrops with pleural effusion, pericardial effusion, ascites, and hepatomegaly. After extensive counseling the family chose expectant management. At 27 weeks’ gestation, a diagnosis of intrauterine fetal death was made. Labor was induced.
Discussion
Parvovirus B19
Parvovirus B19 is a small, single-stranded, nonenveloped DNA virus of the Parvoviridae family. It is the only strain known to be pathogenic in humans. Parvovirus has a predilection for rapidly dividing cells, such as developing red blood cells. Destruction of erythroid progenitor cells leads to hemolysis, red cell aplasia, and impairment of reticulocytosis. After the infection, the aplastic stage lasts approximately 10 days, with complete bone marrow recovery in 2 to 3 weeks.
Epidemiology
Parvovirus infection has a worldwide distribution with a seasonal pattern causing outbreaks most frequently in winter and spring. Commonly passed by respiratory tract secretions from close personal contact, it can also be passed by hand to mouth or by transfusion of infected blood products. The incubation period ranges from 4 to 14 days. Viremia develops approximately 7 days after inoculation and persists for up to 4 days, and a rash appears by day 16. With development of the characteristic exanthem, the primary host is no longer infectious. Previous infection confers immunity.
Clinical Manifestations
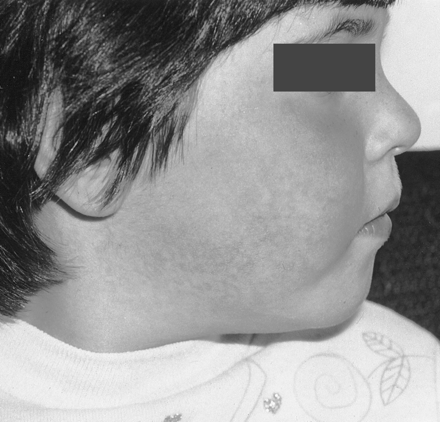
Parvovirus B19 is primarily a disease of school-aged children. The characteristic edematous, lacy, red, malar rash begins on the face (slapped cheeks) (Figure 3) and later spreads to the neck, trunk, buttocks, and extremities. The rash usually subsides in about 10 days and is one of the last clinical signs of infection. The prodromal features include malaise, headache, sore throat, coryza, and low-grade fever. Recurrences are common during the next several months. The patient is not infectious during recurrences. Although most children infected will develop the rash, up to 25% will be asymptomatic.
Photograph showing the slapped-cheek-like rash on the face of a child suffering with fifth disease. (The photograph is provided by Dr. Paul Hoenig from The Children’s Hospital of Philadelphia.)
Seventy-five percent of adults who become infected with parvovirus B19 will be symptomatic. The main symptoms consist of numbness and tingling of the fingers, fatigue, and mild anemia. Arthralgias occur in approximately 80% of adults and can last from days to months. Joints most frequently involved are wrists, hands, ankles, knees, and the interphalangeal joints. The joints are rarely swollen and synovitis is unusual.
Parvovirus B19 has a special affinity for the erythroid system. It affects the final stage of the red cell maturation, causing both hemolysis and red blood cell aplasia. Otherwise healthy adults suffer a mild, asymptomatic anemia. Those with underlying disease or ongoing blood loss can become severely anemic. Immunocompromised patients who are unable to resolve the viral infection can develop chronic bone marrow suppression secondary to prolonged red cell aplasia.
Approximately 50% to 65% of pregnant women are immune at the time of pregnancy. For susceptible pregnant women the risk of infection is high during epidemics and is associated with the level of contact with children. Most infections during pregnancy are due to exposure from a woman’s own children. No significant differences were found in maternal infection from occupational exposures.9 ,10
Although parvovirus has no adverse effects on the healthy pregnant mother, its transplacental transmission to the fetus is an important cause of intrauterine death, abortion, and stillbirth. These outcomes can occur after symptomatic or asymptomatic maternal infection. The transmission rate to fetus is about 33%, and the risk for fetal death is 0% to 9%. The most common clinical manifestation of fetal anemia is nonimmune hydrops, which is observed sonographically as scalp and skin edema, ascites, pleural effusion, placentomegaly, and polyhydramnios. Most cases of hydrops are due to fetal aplastic anemia, which leads to high-output cardiac failure and myocarditis. Parvovirus has been reported to cause approximately 10% of nonimmune fetal hydrops cases, although one study showed no detected case of fetal hydrops in 52 IgM-positive women.9 The mother of any fetus with hydrops should therefore be tested for parvovirus infection, even in the absence of known exposure.
The rate of first trimester abortions is increased in women who are infected with parvovirus. The cause is as yet unclear, but it might be related to the multisystem damage caused by the virus. In the second half of pregnancy, stillbirth in parvovirus B19 infected fetuses can result from the previously listed complications of the infection. Parvovirus B19 also can be an important cause of intrauterine fetal death in the third trimester, and third trimester deaths are often not hydropic.11 Intrauterine death should prompt a woman to consider undergoing serum evaluation for parvovirus antibodies as part of the evaluation of the fetus.12 Tolfvenstam et al11 reported that 15% of cases of intrauterine fetal death were positive for parvovirus B19 in fetal or placental tissues or both.
Although published reports have linked parvovirus B19 infection to congenital malformations in the fetus, a direct relation has not been confirmed. Most postmortem findings were related to the infection, in particular myocarditis and hepatic abnormalities, and it is most likely that the findings of fetal malformations are coincidental.13 Pregnancy termination is not recommended.14 ,15 Infected pregnant women should be cared for by an obstetrician, however, and the diagnostic and treatment options discussed with them.
Laboratory Diagnosis
The diagnosis of acute parvovirus B19 infection can be made most reliably by the finding of virus-specific IgM antibodies or by the isolation of parvovirus DNA detected by nested polymerase chain reaction, dot blot hybridization, and in situ hybridization. A positive IgM finding indicates recent infection and is usually elevated 21 to 24 days after exposure to the virus or 3 to 4 days after the onset of clinical illness. It generally persists for 2 to 3 months, although in some patients it can persist for more than 6 months. IgG is positive 24 to 28 days after exposure or 7 days after clinical infection and remains elevated, indicating lifelong immunity. The finding of both IgM and IgG antibodies indicates an infection that began as recently as 7 days to as long ago as 6 months previously. The sensitivity and specificity for antibody tests can vary. The sensitive polymerase chain reaction for DNA detection might be the best indicator of infection, not only in fetal but also in maternal blood, at least in doubtful cases.16 The virus does not grow well in routine cell culture. Maternal serum α-fetoprotein levels can be elevated in women infected by parvovirus B19. This elevation probably arises from damage to fetal liver cells or infected placental cells.17–19
Management in Pregnancy
Once the acute maternal infection is determined by serologic testing, fetal sonograms should be performed as soon as possible, and weekly follow-up sonography should be continued for 14 to 16 weeks to evaluate for signs of hydrops. If hydrops develops, a decision should be made whether to manage the case expectantly or to perform diagnostic cordocentesis and intrauterine transfusion, as indicated. The risk of fetal death appears to be higher in fetuses managed expectantly than in those managed with intrauterine transfusion. Rodis et al8 recently reported the outcomes of 539 cases of parvovirus-induced hydrops. The study showed survival of 83.5% of hydropic fetuses that were transfused, whereas, in general, nonimmune fetal hydrops has been associated with mortality rates ranging from 50% to 98%. The average time for hydrops resolution is 4 weeks. Antenatal testing (eg, nonstress test, biophysical profile, and contraction stress test) should be performed in the affected fetuses as indicated. The long-term consequences of in utero exposure to parvovirus B19 are unclear. Some studies have shown associations between fetal infection and preterm delivery, severe hyperbilirubinemia, neonatal hepatitis, transfusion-dependent anemia, central nervous system malformations, and neurodevelopmental abnormalities.20 The frequency with which these events occurred has not been established. Most fetuses exposed to maternal parvovirus infection can be expected to have a normal outcome. There is no apparent increase in the frequency of developmental delays in those children.21
A susceptible pregnant woman is most likely to contract parvovirus B19 infection from exposure to her own child, and ill persons are contagious before they develop the characteristic rash. It is not recommended that pregnant women be removed from the workplace during endemic periods. Unfortunately, in many cases, by the time clinical disease is apparent in children, exposure has already occurred. In those cases, the pregnant woman should be tested for acute infection and previous immune status. A seronegative health care worker, pregnant and less than 21 weeks’ gestation, has the same or less risk in the workplace than in the community.22 Because parvovirus infection can also be asymptomatic, any pregnant woman with hydrops fetalis or intrauterine demise should be tested.
There is no vaccine or medicine that prevents parvovirus B19 infection. Frequent hand washing, respiratory precautions, and disposal of used facial tissues are recommended as practical and probably effective methods to reduce the spread of parvovirus. Excluding persons with fifth disease from work, childcare centers, schools, or other settings is not likely to prevent the spread of virus.23 ,24 The value of postexposure prophylaxis with normal immunoglobulin has not been assessed. Intravenous immunoglobulin therapy seems to be effective, however, for the treatment of chronic infection in immunodeficient patient.23
Summary
Infections caused by human parvovirus B19 can result in a wide spectrum of manifestations. In children, parvovirus B19 infection is the cause of the relatively benign disease erythema infectiosum, also known as fifth disease. In healthy adults, it is a common cause of acute arthritis, arthralgias, generalized edema, and vasculitis. In the immunocompromised patient, it can also cause chronic anemia or aplastic anemia. Fetal infection by parvovirus B19 is a common cause of fetal anemia, nonimmune hydrops fetalis, and spontaneous abortion, and it can result in fetal death. Family physicians not only should recognize and manage fifth disease in children but also should educate their pregnant patients about the danger of exposure to this infection. In view of the widespread occurrence of parvovirus B19 and the low incidence of ill effects on the fetus, routine exclusion of pregnant women from the workplace is not recommended. Recent improvements in diagnosing parvovirus infections and intrauterine transfusion have reduced the overall rate of fetal loss after maternal exposure. Transfusion therapy is superior to conservative management in infected fetuses.
- Received for publication December 5, 2001.
- Revision received December 5, 2001.