Abstract
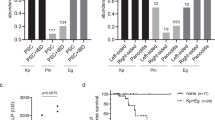
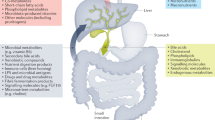
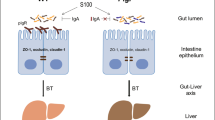
Primary sclerosing cholangitis (PSC) is a chronic inflammatory liver disease and its frequent complication with ulcerative colitis highlights the pathogenic role of epithelial barrier dysfunction. Intestinal barrier dysfunction has been implicated in the pathogenesis of PSC, yet its underlying mechanism remains unknown. Here, we identify Klebsiella pneumonia in the microbiota of patients with PSC and demonstrate that K. pneumoniae disrupts the epithelial barrier to initiate bacterial translocation and liver inflammatory responses. Gnotobiotic mice inoculated with PSC-derived microbiota exhibited T helper 17 (TH17) cell responses in the liver and increased susceptibility to hepatobiliary injuries. Bacterial culture of mesenteric lymph nodes in these mice isolated K. pneumoniae, Proteus mirabilis and Enterococcus gallinarum, which were prevalently detected in patients with PSC. A bacterial-organoid co-culture system visualized the epithelial-damaging effect of PSC-derived K. pneumoniae that was associated with bacterial translocation and susceptibility to TH17-mediated hepatobiliary injuries. We also show that antibiotic treatment ameliorated the TH17 immune response induced by PSC-derived microbiota. These results highlight the role of pathobionts in intestinal barrier dysfunction and liver inflammation, providing insights into therapeutic strategies for PSC.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon request. 16S rRNA sequencing and whole-genome sequencing data have been deposited in the DNA Data Bank of Japan (DDBJ) database with the accession numbers DRA007475 and DRA007476 for 16S rRNA sequencing and PRJDB7545 for whole-genome sequencing. RNA sequencing data have been deposited in the European Genome-phenome Archive (EGA) database with the accession number EGAS00001003332.
References
Lazaridis, K. N. & LaRusso, N. F. Primary sclerosing cholangitis. N. Engl. J. Med. 375, 1161–1170 (2016).
Horsley-Silva, J. L., Carey, E. J. & Lindor, K. D. Advances in primary sclerosing cholangitis. Lancet Gastroenterol. Hepatol. 1, 68–77 (2016).
Hirschfield, G. M., Karlsen, T. H., Lindor, K. D. & Adams, D. H. Primary sclerosing cholangitis. Lancet 382, 1587–1599 (2013).
Dyson, J. K., Beuers, U., Jones, D. E. J., Lohse, A. W. & Hudson, M. Primary sclerosing cholangitis. Lancet 391, 2547–2559 (2018).
O’Toole, A. et al. Primary sclerosing cholangitis and disease distribution in inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 10, 439–441 (2012).
Sasatomi, K., Noguchi, K., Sakisaka, S., Sata, M. & Tanikawa, K. Abnormal accumulation of endotoxin in biliary epithelial cells in primary biliary cirrhosis and primary sclerosing cholangitis. J. Hepatol. 29, 409–416 (1998).
Katt, J. et al. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 58, 1084–1093 (2013).
Loftus, E. V. Jr, Sandborn, W. J., Lindor, K. D. & Larusso, N. F. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm. Bowel Dis. 3, 288–302 (1997).
Loftus, E. V. et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54, 91–96 (2005).
Claessen, M. M. et al. More right-sided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm. Bowel Dis. 15, 1331–1336 (2009).
Karlsen, T. H. & Boberg, K. M. Update on primary sclerosing cholangitis. J. Hepatol. 59, 571–582 (2013).
Sabino, J. et al. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut 65, 1681–1689 (2016).
Kummen, M. et al. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut 66, 611–619 (2017).
Iwasawa, K. et al. Characterisation of the faecal microbiota in Japanese patients with paediatric-onset primary sclerosing cholangitis. Gut 66, 1344–1346 (2017).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Pollheimer, M. J., Trauner, M. & Fickert, P. Will we ever model PSC?—“It’s hard to be a PSC model!”. Clin. Res. Hepatol. Gastroenterol. 35, 792–804 (2011).
Tephly, T. R., Gibbs, A. H. & De Matteis, F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem. J. 180, 241–244 (1979).
Fickert, P. et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 171, 525–536 (2007).
Atarashi, K. et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 358, 359–365 (2017).
Bajer, L. et al. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J. Gastroenterol. 23, 4548–4558 (2017).
Withers, D. R. et al. Transient inhibition of ROR-γt therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nat. Med. 22, 319–323 (2016).
In, J. et al. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stemcell-derived colonoids. Cell. Mol. Gastroenterol. Hepatol. 2, 48–62 (2016).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Shneider, M. M. et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature 500, 350–353 (2013).
Russell, A. B., Peterson, S. B. & Mougous, J. D. Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 12, 137–148 (2014).
Siu, L. K., Yeh, K. M., Lin, J. C., Fung, C. P. & Chang, F. Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 12, 881–887 (2012).
Murray, B. E. The life and times of the Enterococcus. Clin. Microbiol. Rev. 3, 46–65 (1990).
Wiest, R., Lawson, M. & Geuking, M. Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 60, 197–209 (2014).
Llorente, C. & Schnabl, B. The gut microbiota and liver disease. Cell. Mol. Gastroenterol. Hepatol. 1, 275–284 (2015).
Miele, L. et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009).
Steffen, E. K., Berg, R. D. & Deitch, E. A. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J. Infect. Dis. 157, 1032–1038 (1988).
Tang, R. et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut 67, 534–541 (2018).
Jiang, F., Waterfield, N. R., Yang, J., Yang, G. & Jin, Q. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15, 600–610 (2014).
Alcoforado Diniz, J., Liu, Y. C. & Coulthurst, S. J. Molecular weaponry: diverse effectors delivered by the type VI secretion system. Cell. Microbiol. 17, 1742–1751 (2015).
Spadoni, I. et al. A gut–vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834 (2015).
Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science 359, 1156–1161 (2018).
Tabibian, J. H. et al. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis—a pilot study. Aliment. Pharmacol. Ther. 37, 604–612 (2013).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Colombel, J. F., Sendid, B., Jouault, T. & Poulain, D. Secukinumab failure in Crohn’s disease: the yeast connection? Gut 62, 800–801 (2013).
McGeachy, M. J. GM-CSF: the secret weapon in the TH17 arsenal. Nat. Immunol. 12, 521–522 (2011).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V. K. IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Gaffen, S. L., Jain, R., Garg, A. V. & Cua, D. J. The IL-23–IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600 (2014).
Xiao, S. et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 40, 477–489 (2014).
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838 (2016).
Mihara, E. et al. Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/alpha-albumin. eLife 5, e11621 (2016).
Nishijima, S. et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 23, 125–133 (2016).
Jackson, C. R., Fedorka-Cray, P. J. & Barrett, J. B. Use of a genus- and species-specific multiplex PCR for identification of enterococci. J. Clin. Microbiol. 42, 3558–3565 (2004).
Stankowska, D., Kwinkowski, M. & Kaca, W. Quantification of Proteus mirabilis virulence factors and modulation by acylated homoserine lactones. J. Microbiol. Immunol. Infect. 41, 243–253 (2008).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Kuczynski, J. et al. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinformatics 36, 10.7.1–10.7.20 (2011).
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C. & Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200 (2011).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Tsuda, A. et al. Influence of proton-pump inhibitors on the luminal microbiota in the gastrointestinal tract. Clin. Transl Gastroenterol. 6, e89 (2015).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Ojiro, K. et al. MyD88-dependent pathway accelerates the liver damage of concanavalin A-induced hepatitis. Biochem. Biophys. Res. Commun. 399, 744–749 (2010).
Hayashi, A. et al. A single strain of Clostridium butyricum induces intestinal IL-10-producing macrophages to suppress acute experimental colitis in mice. Cell Host Microbe 13, 711–722 (2013).
Ondov, B. D. et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 17, 132 (2016).
Brisse, S. et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J. Clin. Microbiol. 51, 4073–4078 (2013).
Pan, Y. J. et al. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS ONE 8, e80670 (2013).
Acknowledgements
We thank S. Chiba, S. Shiba, R. Morikawa, T. Katayama, A. Ikura, Y. Mikami and T. Sujino (Division of Gastroenterology and Hepatology, Keio University) for technical assistance and critical reading of this manuscript. This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid (C) 16K09374 and (A) 15H02534, the Advanced Research and Development Programs for Medical Innovation (AMED-CREST; 16gm1010003h0001), the TAKEDA Science Fund, Ezaki Glico Co. Ltd and Keio University Medical Fund. K.H. was funded through AMED LEAP under grant number JP17gm0010003. A. Yoshimura was supported by the JSPS KAKENHI Grant-in-Aid (S) JP17H06175, Challenging Research (P) JP18H05376 and AMED-CREST JP18gm0510019 and JP18gm1110009.
Author information
Authors and Affiliations
Contributions
N.N. and T.K. designed the project. N.N., N.S., R.A., K.M., T.T., Takahiro S., Y.K., P.-S.C., N.T., Akihiro Y., M.K. and H.A. performed the experiments. W.S. and M.H. performed the bacterial sequence, microbiome analyses and contributed to data discussions. K.A., S.N. and K.H. provided essential materials and contributed to data discussions. N.N., N.K., M.S., Akihiko Y., Toshiro S. and T.K. interpreted the experimental data. N.N., N.K. and Toshiro S. wrote the manuscript. T.K. critically revised the manuscript and supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–8.
Supplementary Table 1
Demographic characteristics of study subjects.
Supplementary Table 2
Fpkm values in RNA-seq analysis.
Supplementary Table 3
Phylogenetic relationship among K. pneumoniae strains used in this study.
Supplementary Table 4
Genes positively correlated with the epithelial pore-forming capacity.
Rights and permissions
About this article
Cite this article
Nakamoto, N., Sasaki, N., Aoki, R. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol 4, 492–503 (2019). https://doi.org/10.1038/s41564-018-0333-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0333-1