Abstract
Several intraocular infections can present with protean manifestations posing major diagnostic and management challenges. Infections such as tuberculosis, dengue and chikungunya fever have continued to remain major endemic diseases that are associated with uveitis in the Asia Pacific region. These entities often require a high index of clinical suspicion and laboratory analysis including assays of ocular fluids and/or tissues for confirmation of the diagnosis. Infectious uveitis caused by tuberculosis, dengue and chikungunya can present with characteristic clinical features and imaging findings on ancillary investigations; that may provide clue to the early diagnosis. Use of modern imaging modalities such as enhanced-depth imaging optical coherence tomography, optical coherence tomography angiography and ultra-wide field fundus photography greatly aid in the evaluation of these conditions. In the current review, we have discussed the epidemiology, clinical phenotypes, imaging characteristics, diagnosis and management of uveitis caused by tuberculosis, dengue and chikungunya.
摘要
眼内感染的临床表现多种多样, 是目前诊断和治疗的主要挑战。结核病, 登革热和基孔肯雅热感染仍是亚太地区与葡萄膜炎相关的主要地方性疾病。这些感染临床需要高度重视和实验室检查, 包括眼部液体和或组织检查以确诊。由结核病, 登革热和基孔肯雅病引起的传染性葡萄膜炎可能具有一些特征性的临床表现和特殊的影像学表现, 这些都可为早期诊断提供线索。现代成像技术的应用, 包括光学相干断层扫描增强深度成像模式、光学相干断层扫描血管造影和超广角眼底成像, 极大地提高了诊断和评估传染性葡萄膜炎准确性。在这篇综述中, 我们讨论了由结核病, 登革热和基孔肯雅热引起的葡萄膜炎的流行病学, 临床表型, 影像学特征以及诊断和治疗。
Similar content being viewed by others
Introduction
A number of infectious agents, including a host of bacteria, viruses, fungi, and parasites lead to ocular inflammation with development of various chorioretinal manifestations. These infections are especially common in Asia Pacific countries and other developing countries of the world, unlike western countries where autoimmune uveitis is the most common form [1,2,3]. An early diagnosis is imperative for the initiation of specific therapy that would help in preventing the severe visual loss.
Since several decades, tuberculosis (TB) has remained endemic in Asian countries with India, with a large population size, accounting for nearly 20% of the global tuberculosis burden. TB is also common in other countries of the Pacific region (Singapore, Malaysia, Bangladesh, and Nepal, among others), and Island countries such as Fiji [4,5,6,7]. Currently, the diagnosis of TB uveitis is a conundrum because the only way to determine tubercular etiology is using a conglomerate of clinical signs, radiologic findings, and immunologic tests.
Certain viral infections such as dengue and chikungunya are highly endemic in the Asia Pacific countries. This may be attributed to the conducive weather and abundance of the arthropod vectors in this part of the world [8,9,10,11]. Due to various contributory factors such as overcrowding, lack of hygiene, excessive rainfall, and poor outreach measures, frequent outbreaks of dengue and chikungunya fevers are common especially in the monsoons. The most common clinical presentation of these viral illnesses is development of high-grade fevers, rash, arthralgia, low platelets, and muscle/bone pain. However, both dengue and chikungunya may result in severe sight-threatening ocular inflammation that may lead to permanent visual disability.
In this index review, infectious uveitis (namely TB, dengue, and chikungunya) that continue to plague Asian countries have been described. A brief update on the epidemiological patterns of these conditions, along with their clinical features, imaging characteristics, and management has been provided. Illustrative case examples have been provided to give the reader insights into the challenges encountered while managing these patients.
Intraocular tuberculosis
TB is a leading infectious cause of morbidity and mortality and Asian countries such as India and China contribute significantly toward the global disease burden. TB has been declared as a global emergency by the World Health Organization (WHO). Nearly one-third of the world’s population is infected by Mycobacterium tuberculosis [5,6,7]. Intraocular TB (IOTB) represents an extrapulmonary form of the disease. Therefore, though rare, IOTB is an important cause of uveitis in both developed and developing countries.
An update on epidemiology of tuberculosis in Asia
TB is highly prevalent in Asian countries. Estimates by the WHO suggest that 4.9 million prevalent cases (one-third of the world’s burden of TB) are found in the South-East Asia Region. High number of cases, including extensively drug-resistant TB have been reported from Bangladesh, India, Indonesia, Myanmar, and Thailand [12]. The WHO statistics for India for 2016 give an estimated incidence of 2.79 million cases of TB. India also has more than a million “missing” cases every year that are not notified and most remain either undiagnosed or unaccountably and inadequately diagnosed and treated in the private sector [13].
Based on the diagnostic criteria used, the incidence of IOTB in India has varied from 0.6% of all uveitis patients in South India (1995) [14] to 10.1% in North India (2004) [15]. In 2017, a report from a major tertiary care center in North India reported that 23% cases of infectious uveitis were attributed to TB [16]. This finding was supported by a study from South India in the same year [17]. Similarly, in Sri Lanka, IOTB was responsible for significant number of patients with posterior uveitis (11%), intermediate uveitis (8%), and panuveitis (11%) [18]. In Philippines (2017), IOTB accounts for more than 25% cases of infectious uveitis, more common than toxoplasmosis. Taiwan reported TB as the most common cause of infectious uveitis (9%) [19]. A study from Singapore also reported that TB was the most common cause of panuveitis, especially among Malays and Indians [20].
TB also forms an important cause of infectious uveitis in children. In a study on pediatric uveitis from North India, TB was identified in 15% cases of infectious uveitis [21]. Out of 20 children diagnosed with intermediate uveitis from South India, 9 were diagnosed with TB in 2017 [22].
Clinical presentation of intraocular tuberculosis
The choroid is the most commonly affected structure in IOTB. Posterior uveitis is the most common form of involvement in IOTB [4, 23, 24]. It is well known that IOTB can have protean clinical manifestations leading to a diagnostic challenge. It can have varied presentations such as granulomatous uveitis and may mimic several inflammatory and non-inflammatory conditions such as tumors, macular degeneration, as well as non-infectious autoimmune uveitic entities [13]. Moderate to severe visual impairment can occur in 42% patients with IOTB, especially in cases of posterior and panuveitis. Therefore, it is relevant to understand the common clinical presentations of the disease.
Selected observations from the COTS-1: The Collaborative Ocular Tuberculosis Study-1 (COTS-1) is a recently completed multicenter retrospective collaborative study between 30 uveitis centers across the world with participation of more than 40 uveitis experts around the world. COTS-1 was a big data analysis that studied the current practice of IOTB worldwide. The study consisted of 962 patients most of whom were of Asian ethnicity (74.4%) [11]. The broad aims of the study were as follows: to determine the global epidemiological profile of IOTB; how do experts diagnose and manage IOTB; what are the treatment outcomes of IOTB.
A total of 945 patients (1485 eyes) diagnosed with IOTB from 2004 to 2014 were included in this study. The COTS-1 showed that individuals with Asian ethnicity (both native and immigrants) are at a high risk of developing features of IOTB. Based on the anatomical location of uveitis, COTS-1 report showed that TB serpiginous-like choroiditis (which represents one of the most characteristic manifestation of the disease) was the most prevalent phenotype in the Asia Pacific region, whereas it was much less prevalent in the West [25]. The common phenotypic variants reported with IOTB included SLC (46.1%), choroidal tuberculomas (13.5%), and multifocal choroiditis (9.4%) [25]. Presumed TB retinal vasculitis was seen in 251 out of 945 patients, most commonly Asians (71%) [26].
The following section summarizes the various forms of IOTB and highlights their clinical and imaging features.
Choroidal tubercles
Choroidal tubercles are the most common clinical manifestations among patients with systemic TB [23, 27]. Choroidal tubercles form as a result of hematogenous dissemination of the bacilli from pulmonary and other extra-pulmonary sites. These tubercles may be unilateral or bilateral, solitary, or multiple (but usually ≤5 in number), discreet grayish-white to yellowish subretinal lesions with indistinct borders. Choroidal tubercles are usually located in the posterior pole or mid-periphery [27].
On fluorescein angiography (FA), choroidal tubercles are hypofluorescent in the dye transit and become hyperfluorescent in the late frames. Optical coherence tomography (OCT) is useful in confirming the presence of a choroidal tubercle.
Choroidal granulomas/subretinal abscess
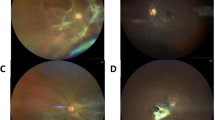
Solitary large choroidal granuloma may present as an elevated yellowish subretinal mass lesion with exudative retinal detachment and fluid (Fig. 1). A choroidal granuloma may clinically resemble non-inflammatory conditions such as central serous chorioretinopathy, choroidal metastases, melanoma of the choroid, and other non-uveitic entities such as age-related macular degeneration [4, 23, 27, 28]. The subretinal abscesses are more yellowish in color than a small choroidal granuloma and may have overlying retinal hemorrhages [29].
Figure shows multimodal imaging of a 55-year-old female who presented with diminuition of vision in the left eye. There was presence of a large subretinal lesion with overlying hemorrhages (white arrows) and exudative fluid (a). Swept-source optical coherence tomography (SS-OCT) (b) B-scan passing through the lesion showed a large choroidal granuloma (asterisk), retinal edema, as well as vitritis (arrowhead). Another OCT B-scan shows presence of retinal pigment epithelial elevation due to choroidal granuloma (asterisk), vitreous cells (arrowhead), and subretinal fluid (white arrow) (c). Fluorescein angiography (FA) in the early phase showed early hyperfluorescence with areas of blocked fluorescence due to overlying hemorrhages (d). In the late phase, FA showed intense hyperfluorescence with peripheral leakage suggestive of an inflammatory choroidal lesion (e). The patient was diagnosed with tubercular choroidal granuloma due to positive laboratory tests
On FA, the lesions show early hypofluorescence and late hyperfluorescence. There may be blocked fluorescence due to the overlying hemorrhages. On ICGA, subretinal abscesses appear hypofluorescent throughout the early as well as late phase [30,31,32]. OCT is useful in detecting exudative retinal detachment associated with choroidal granulomas [28, 33]. Enhanced-depth imaging OCT (EDI-OCT) shows choroidal changes that correlate well with the findings on indocyanine green angiography (ICGA). A recent study showed that all choroidal granuloma lesions generated an increased transmission of the OCT signal towards the sclera [34]. Compared with small lesions, large granulomas were more likely to be full-thickness, round-shaped, with defined margins, lower reflective than the surrounding structures, and with a homogenous internal pattern. Granulomas in patients affected by TB-related uveitis were more likely to have a lobulated shape and non-homogeneous internal pattern [34, 35].
Serpiginous-like choroiditis
Tubercular serpiginous-like choroiditis (TB SLC) is a characteristic entity which is very common among young to middle-aged adults from TB-endemic areas in the Asia Pacific region [23, 36, 37]. TB SLC was first described by Gupta et al. in 2003 in 11 eyes of 7 patients. All the patients in the series had strongly positive tuberculin skin test and lesions on chest radiography. For the first time, the authors concluded that these lesions showed a good response to anti-tubercular therapy [38]. Following this report, there were several reports from different parts of the world that associated SLC with TB [39,40,41]. In a recently published report, Mycobacterium tuberculosis DNA was isolated in an Asian Indian male with SLC lesions on vitreous biopsy. Chorioretinal biopsy sample showed granulomatous inflammation with central choroidal necrosis and disruption of the outer retina/retinal pigment epithelium [42].
TB SLC can be distinguished from autoimmune garden variety of choroiditis by its subtle differences. Unlike autoimmune serpiginous choroiditis, TB SLC occurs at a younger age, can be associated with mild vitritis and is commonly bilateral. It may have different morphological patterns:
-
1.
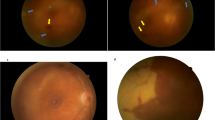
Multifocal pattern: This pattern is characterized by the presence of multifocal discreet lesions that are yellowish-white in color, measuring a maximum of one disc diameter in size with well-defined margins and slightly raised edges [36]. These progress over a few weeks and gradually become confluent (Figs. 2 and 3) [36, 43].
Fig. 2 Fundus photograph of a patient with tubercular serpiginous-like choroiditis (TB SLC) shows presence of yellowish serpentine choroiditis lesions in the posterior pole with fuzzy edges and ill-defined margins (a). The autofluorescence imaging shows mixed hyper-autofluorescent (within the lesion) and hypo-autofluorescence of the lesions suggestive of activity (b). Early phase of the combined fluorescein angiography (FA) and indocyanine green angiography (ICGA) shows hypofluorescence of the active lesions (c), and in the late phase (d), the lesions are hyperfluorescent on FA and continue to remain hypofluorescent in the ICGA. The optical coherence tomography (dense scan) passing through the choroiditis lesions show outer retinal disruption (white arrowheads), choriocapillaris ischemia, and disruption of the photoreceptor layer (white dashed square) (e)
Fig. 3 Figure shows fundus imaging of a 35-year-old Asian Indian male who presented with characteristic choroidal lesions in the right eye. Fundus photographs (a–c) shows presence of multiple yellowish-white multifocal choroiditis lesions with fuzzy appearance, ill-defined margins and early pigmentary changes suggestive of active serpiginous-like choroiditis. The patient had a necrotic tuberculin skin test and few subcentimetric lymph nodes in his chest. Fundus autofluorescence imaging (d, e) shows hyper-autofluorescence within the lesions along with hypo-autofluorescence of the superior lesions. Optical coherence tomography angiography en face scan passing through the choriocapillaris slab (f) shows presence of dark flow deficit areas suggestive of choriocapillaris hypoperfusion, which is seen in active tubercular choroiditis
-
2.
Placoid pattern: The diffuse placoid pattern of TB SLC presents with a large plaque-like lesion and an active serpentine edge [44]. The edges are yellowish and elevated whereas the center of the lesion is less elevated with pigmentary changes. This pattern suggests healing process in the center of the lesion with activity in the periphery of the lesion. Choriocapillaris atrophy is commonly seen in the center of the lesion with visible underlying choroidal vessels [36, 43].
-
3.
Mixed pattern: This pattern of TB SLC presents with overlapping features in opposite eyes.
Imaging features of TB SLC
Fluorescein angiography and indocyanine green angiography: There is a uniform agreement in the literature on the appearance of active and inactive lesions of TB SLC on FA and ICGA. Multifocal TB SLC lesions are hypofluorescent in the early phase with late hyperfluorescence on FA. The advancing edge of the lesions may show early hypofluorescence with late hyperfluorescence. On the other hand, in placoid TB SLC lesions, the center of the lesion shows mixed hyperfluorescence while the advancing edge shows early hypofluorescence with late hyperfluorescence on FA. On ICGA, both the lesions remain hypofluorescence from early to late phase during the active stage [36, 43, 45, 46].
Fundus autofluorescence (FAF) features of TB SLC: FAF features of TB SLC are relevant in the understanding of the healing patterns of the disease. Based on the FAF appearance of the lesions, our group has shown that in the early active disease, the lesions appear predominantly hyper-autofluorescent on FAF (stage 1). With progressive healing, FAF shows a combination of hypo- and hyper-autofluorescence signals, where the distinct hypo-autofluorescent (inactive) rim surrounds the hyper-autofluorescent (active) center (stage 2). As the healing process continues, there is a progressive increase in the hypo-autofluorescence of the lesion with an intermixed hyper-autofluorescent stippled pattern (stage 3). A resolved fully resolved/healed lesion shows uniform hypo-autofluorescence (stage 4) [45, 47, 48].
A case report by Gupta and Biswas in 2014 [49] defined a sequential pattern of FAF during the entire course of TB SLC, i.e., evolution, progression, and healing. In the evolution phase, they showed that the lesions were hyper-autofluorescent. They also noticed faint hyperautofluorescence extending over a large area that was predictive of future extent of the lesion. The authors defined progression when the advancing edge showed more hyperautofluorescence. During healing, there was sharpening of hyper-autofluorescent borders, and few specks of hyperautofluorescence were seen within the hypo-autofluorescent lesion [49]. Piccolino et al. also described similar FAF features; however, they suggested that even early active lesions of TB SLC may have hypo-autofluorescence within the lesion [50]. Carreno et al. have described a similar pattern of FAF findings in TB SLC: active inflammation, transitional, and inactive inflammation characterized by increasing hypo-autofluorescence as the lesions begin to heal [51].
In a study by Khanamiri and Rao, during the active stage, the authors have stated that there may be no FAF abnormalities in the first 1–2 days after clinical presentation. Subsequently, acute lesions show hyper- and hypoautofluorescence patches with sharp margins. After 2 weeks, the lesions show granular/speckled pattern of autofluorescence. Healed lesions appear uniform hypo-autofluorescent [46].
Ultra-wide field imaging of TB SLC: The relatively new ultra-wide field (UWF) imaging technique allows 200 degrees of the retina to be captured in a single image thereby allowing simultaneous capture of all retinal lesions in a single frame. As shown by Aggarwal et al., UWF imaging is a very useful tool in the management of IOTB as it helps to capture peripheral areas of retinal non-perfusion, active retinal vasculitis, neovascularization, and choroiditis lesions which would otherwise be missed on conventional fundus imaging [52,53,54]. UWF imaging revealed additional TB SLC lesions in 39 out of 44 eyes (88.6%) compared to conventional imaging. It has been shown to provide additional information in 90.9% of the eyes with TB posterior uveitis and affected management decisions in over 45% of the eyes [52].
OCT features of TB SLC: Various studies have shown the role of OCT in detecting retinochoroidal changes in TB SLC. In the active stage of the disease, OCT B-scans passing the active edge of TB SLC lesion show a localized, fuzzy area of hyperreflectivity in the outer retinal layers involving the RPE, ellipsoid and myoid zone, external limiting membrane (ELM), and the outer nuclear layer (ONL) with no increased backscattering from the inner choroid. As the lesions heal, there is disappearance of the hyperreflective fuzzy areas which are replaced by irregular, hyperreflective knobbly elevations of the outer retinal layers. The RPE, ellipsoid and myoid zones, and the ELM cannot be distinguished at this stage [24, 43, 45, 55].
Rifkin et al. have shown that EDI-OCT is very useful in detecting active choroidal infiltration with RPE elevation in patients with TB SLC. The authors proposed that this finding may be useful in distinguishing TB SLC from the autoimmune garden variety of serpiginous choroiditis [56].
OCT angiography in TB choroiditis: The novel technique of OCT angiography has recently shown novel findings that greatly aid in understanding the pathology and sequelae of chorioretinal changes in TB SLC. OCT angiography provides high-resolution imaging of the choriocapillaris in different stages of this disease. In the acute stage, Mandadi et al. have shown alterations in choriocapillaris flow (flow deficit) that may progress to atrophy during the inactive stage (Fig. 3). Morphologic information obtained from OCT angiography images correlates well with and supplements other imaging techniques such as ICGA and EDI-OCT [57].
Recently, OCT angiography has also been shown to be useful in detecting novel type 1 choroidal neovascular (CNV) lesions among patients with TB SLC. Aggarwal et al. demonstrate that type 1 CNV can lead to significant visual loss even in the healed stages of the disease. OCT angiography is useful even in cases where conventional multimodal imaging, including FA and OCT, fail to make a definitive diagnosis [58].
Presumed tubercular retinal vasculitis
Presumed TB retinal vasculitis is characterized by peripheral occlusive retinal periphlebitis with inflamed retinal vessels and features of distal retinal ischemia such as cotton-wool spots or lack of blood flow on fundus imaging. TB vasculitis can present with sequelae such as peripheral retinal neovascularisation and recurrent vitreous hemorrhage leading to tractional retinal detachment and/or macular scarring. This condition may mimic other causes of retinal vasculitis such as other infective causes (toxoplasmosis, viral retinitis, syphilis, toxocariasis, among others) and non-infective causes such as birdshot chorioretinopathy, systemic vasculitides, among others [26, 59, 60].
Eales’ disease is a condition characterized by occlusive vasculitis and development of retinal neovascularization. Biswas et al. have been instrumental in providing an update on the current understanding and etiopathogenesis of this condition [61, 62]. Eales’ disease is presently thought to be due to hypersensitivity reaction to tubercular proteins. In a study, significant number of patients operated for epiretinal membrane were positive for Mycobacteria when tested by polymerase chain reaction (PCR) [63]. Verma et al. have shown presence of Mycobacterium tuberculosis DNA in an enucleated eyeball using nested PCR technique [64]. More recently, our group has shown that Mycobacterium tuberculosis genome was present in more than 50% vitreous fluid samples of patients with Eales’ disease with a significant bacillary load [65]. Therefore, there is increasing evidence that Eales’ disease could indeed represent TB vasculitis especially in endemic Asian countries, and these patients must be thoroughly investigated for evidence of TB infection in the body [61].
There is no definitive method of establishing TB as the etiology of retinal vasculitis. Therefore, these cases are considered to be presumed tubercular in nature. Usually, the diagnosis of TB retinal vasculitis is suspected by the treating uveitis experts by considering various clinical, immunological, and radiological criteria, and excluding other infectious and non-infectious known etiologies [26, 66, 67]. Among individuals with Asian ethnicity, majority of the patients with TB retinal vasculitis will present with an occlusive disease. It is pertinent to note that most Caucasian patients may not have an occlusive disease [26]. Such differences may be related to the genetic differences (e.g., HLA) between these ethnicities of patients.
While there is a lack of consensus on the diagnostic criteria and management of TB retinal vasculitis, this condition has shown good anatomic and visual response with prompt ATT and/or steroid therapy in smaller studies [59, 68].
Unusual clinical phenotypes
Rarely, in endemic countries, IOTB may present with uncommon phenotypes such as panophthalmitis and endophthalmitis, which may mimic as ocular tumors [69, 70]. Tuberculous optic neuropathy is also uncommon, and may manifest as papillitis, neuroretinitis, and optic nerve tubercle [71,72,73]. Optic nerve head tubercle may present as an elevation at the optic nerve head with surrounding exudation and fluid. Choroidal involvement in IOTB may also have rare phenotypic presentations. In the COTS-1, IOTB was diagnosed in patients with ampiginous choroiditis, acute posterior multifocal placoid pigment epitheliopathy (APMPPE), and other types of choroiditis that did not fit into any of the classical descriptions [25]. Such forms of choroidal involvement in IOTB has not been previously reported.
TB panuveitis may be confused with other forms of granulomatous panuveitis. Many patients may require extensive clinical, laboratory, and imaging workup, including invasive microbiological and histopathological evidence in order to establish a diagnosis. In a case report published by our group in 2016, we describe a monocular Asian Indian female who presented with significant panuveitis which was misdiagnosed as sympathetic ophthalmia. The correct diagnosis could be established only when the non-seeing phthisical fellow eye was enucleated and careful histopathological examination revealed presence of acid-fast bacilli (AFB) on Ziehl–Neelsen staining, tubercular granulomas, and the sample tested positive for TB on PCR [74].
It must be kept in mind that in rare phenotypic presentations, the diagnosis of TB may be presumptive, based on a constellation of clinical, radiological, and laboratory findings. It must be emphasized that in a lot of patients, unequivocal evidence of the infection is often unavailable.
Diagnosis of intraocular tuberculosis
The diagnosis of IOTB is indeed challenging. Till date, there are no randomized controlled trials that have defined the diagnosis of IOTB. Furthermore, there are no consensus guidelines amongst the uveitis experts world over regarding the diagnosis of IOTB. The criteria that have been applied for diagnosing IOTB by various studies include [4, 23, 45, 75]:
-
1.
Clinical signs suggestive of IOTB including:
-
a.
Anterior uveitis: Granulomatous or non-granulomatous, iris nodules, ciliary body granuloma.
-
b.
Intermediate uveitis: Granulomatous or non-granulomatous with exudates in the pars plana or peripheral uvea, with/without snow balls.
-
c.
Posterior and Panuveitis: choroidal tubercle, choroidal granuloma, subretinal abscess, serpiginous-like choroiditis.
-
d.
Other features such as retinal vasculitis, neuroretinitis, optic neuritis, endophthalmitis, panophthalmitis, scleritis.
-
a.
-
2.
Exclusion of other uveitic entities based on clinical manifestations of disease and regional epidemiology.
-
3.
Investigations documenting the mycobacteria or its genome:
-
a.
Demonstration of AFB by microscopy or culture of Mycobacterium tuberculosis from ocular fluid
-
b.
Positive polymerase chain reaction from ocular fluid for IS 6110 or other conserved sequences in mycobacterial genome
-
c.
Evidence of confirmed active extra-pulmonary TB (by microscopic examination or culture of a tissue sample from the affected tissue)
-
a.
-
4.
Corroborative investigations such as:
-
a.
Positive Mantoux test
-
b.
Interferon Gamma Release Assay (IGRA) such as QuantiFERON TB Gold
-
c.
Evidence of healed or active TB on chest radiography
-
a.
There is no single gold standard test available for diagnosing IOTB. Tests such as Mantoux test and interferon gamma release assays may be highly positive in some centers (especially in Asian countries) due to endemicity of TB in the region. Therefore, it is necessary to develop a prospectively derived clinical risk score that will help to improve the diagnosis and management of IOTB.
Therapies for intraocular tuberculosis
Standard therapy for intraocular tuberculosis
Anti-tubercular therapy (ATT) consists of a combination of four drugs namely isoniazid, rifampin, ethambutol, and pyrazinamide. These four agents constitute the first-line agents and are started empirically in patients with systemic TB. The four agents are given in the following doses: isoniazid (5 mg/kg/day), rifampicin (10 mg/kg/day), ethambutol (15 mg/kg/day), and pyrazinamide (20–25 mg/kg/day) along with pyridoxine (vitamin B6) (10 mg/day).
In 2008, Bansal et al. reviewed the role of ATT in the treatment of active TB uveitis in a large retrospective interventional series (n = 360). In this study, the authors observed that among the 216 patients who received ATT, the recurrences of uveitis substantially decreased compared to 144 patients who did not receive ATT (15.74% versus 46.53%; p < 0.001). Therefore, the authors speculated that since the disease occurs due to a hypersensitivity reaction, ATT may have an important role in treating latent TB infection in the body, eliminating future recurrences of IOTB [76]. While the study by Bansal et al. was performed in a highly endemic region of world for TB, i.e. India, Agrawal et al. published a retrospective study highlighting the role of ATT in a low endemic country (UK) in 2015. In this study, patients who received long-term treatment with ATT showed reduced recurrence of the disease [77].
In 2016, a major review and systematic analysis of 28 studies (a total of 1917 patients) published by Kee et al. reviewed the role of ATT for IOTB. The study results showed that among patients who received ATT, the nonrecurrence of inflammation was observed in pooled estimate of 84%. While the study results showed benefit of ATT in reducing recurrences, there were limitations due to lack of a control group and non-standard recruitment and treatment criteria [78].
The standard therapy for IOTB as per the recommended guidelines consists of four drug regimen mentioned above. Ethambutol and pyrazinamide are stopped after a period of 2 months. There is regular monitoring of liver function tests when a patient is started on ATT.
Thus far, there is no definite guideline on the duration of ATT in patients with IOTB. Agrawal et al. have shown beneficial results in patients who receive ATT for ≥9 months [77]. In a study from Singapore by Ang et al., 186 patients with IOTB were included, of whom 46 received more than 6 months of ATT. The authors showed that patients who completed >9 months ATT were less likely to develop recurrences compared with those not treated with ATT (p = 0.027) [79]. Till date, however, there is no common consensus among uveitis experts regarding the regimen and duration of ATT, as shown in the COTS-1 study [25].
Managing inflammation in intraocular tuberculosis—oral corticosteroids
There is no uniform recommendation regarding the use of concomitant corticosteroids along with ATT in IOTB. Corticosteroids are employed to reduce the intraocular inflammation since IOTB is hypothesized to occur due to type IV hypersensitivity reaction to tubercular proteins. Oral corticosteroids may also play a role in reducing inflammatory macular edema [80]. However, unlike in the case of meningeal and pericardial TB where there are clear guidelines supporting the use of systemic corticosteroids, their use in IOTB is considered to be controversial by certain authors [81].
Various series have reported the use of oral corticosteroids along with ATT as a combination (oral prednisolone 1 mg/kg/day) for IOTB with favorable control of inflammation [23, 36, 37, 68, 76, 77, 82]. Corticosteroids can be tapered over the next 6–12 weeks depending upon the severity of inflammation and occurrence of paradoxical worsening of the disease [83]. Certain authors favor the use of corticosteroids only when the lesions are involving or threatening the macula in order to decrease macular scarring [84].
Paradoxical worsening
One of the most clinically intriguing features of TB SLC is the paradoxical worsening of the disease after initiation of anti-tubercular therapy (ATT). The worsening of these lesions occurs due to a combination of factors including enhanced delayed hypersensitivity of the host, decreased suppressor mechanisms, and increased exposure to the mycobacterial antigens or a response to mycobacterial antigens such as tubercular proteins [37, 85, 86]. Cheung and Chee have described a case of a 77-year-old woman with biopsy-proven TB cervical lymphadenitis who developed choroiditis in one eye after initiation of ATT. The authors identified mycobacterial DNA from the vitreous tap using polymerase chain reaction, and a diagnosis of paradoxical worsening following ATT was made [87]. In a case series by Basu et al., 4 patients with IOTB (including 1 patient with intermediate uveitis; 1 patient with TB granuloma, and 2 patients with TB SLC) who were started on ATT developed paradoxical worsening (appearance of new lesions or worsening of existing lesions). Therefore, paradoxical worsening is a major challenge in the management of IOTB [82].
With the recent introduction of ultra-wide field (UWF) retinal imaging, the detection rates of paradoxical worsening have significantly increased. Using conventional imaging, paradoxical worsening was reported in 14% patients after 2 to 6 weeks of initiation of ATT [37]. With UWF imaging, paradoxical worsening may be observed in over 36% patients after initiation of ATT [52, 88, 89]. In a series by Agarwal et al., OCT angiography was shown to be very useful in the detection of choriocapillaris alterations in patients of TB SLC developing paradoxical worsening [88]. Patients who develop paradoxical worsening may require increase in the dosage of corticosteroids, or addition of intravenous methylprednisolone pulses. Topical steroids may be employed in cases with anterior segment inflammation. Systemic immunosuppressants and steroid sparing agents such as azathioprine may be added as and when required depending on the discretion of the treating uveitis specialist.
Novel local and systemic therapies
In order to improve the outcomes of IOTB by reducing the systemic corticosteroid-related adverse effects in severe or long-standing inflammation, and address various challenges such as paradoxical worsening of the disease, a number of alternative therapeutic strategies have been tried. Intravitreal injection of depot steroid (dexamethasone implant, Ozurdex ®) has been employed in the management of TB multifocal serpiginoid choroiditis [90, 91]. In a case report by Fonollosa et al., Ozurdex implant was shown to be useful in continuous progression of the lesions despite ATT and oral corticosteroids [90]. Similarly, Jain et al. showed that Ozurdex implant is useful in cases where the disease continues to progress, or there is intolerance to oral corticosteroids [91]. In a larger series by Agarwal et al., 19 eyes of 17 patients with IOTB (including intermediate uveitis, retinal vasculitis, and TB SLC) received Ozurdex implant with favorable resolution of inflammation, and improvement in visual acuity [92].
Intravitreal methotrexate has also been employed in the management of IOTB. In a series by Julian et al., 3 eyes of 2 patients with active presumed TB choroiditis received intravitreal methotrexate due to progressive macular threatening disease. In all 3 eyes, healing of choroidal lesions without any adverse event occurred within 1 month of the injections [93]. Sahin et al. published a similar favorable experience with intravitreal methotrexate in 2 cases of IOTB [94].
Interferons (IFN) are a group of low molecular weight polypeptides secreted by activated immune cells and possess high activity and various functions. IFNs alpha/beta signaling by the retinal pigment epithelium has been linked to high Mycobacterium tuberculosis disease activity in patients with active disease, leading to inhibition of the outgrowth of intracellular mycobacteria [95]. In a series of 12 eyes (6 patients) by Invernizzi et al., IFN-alpha 2a was employed in the management of presumed chronic TB uveitis patients in whom the uveitis was recurrent upon tapering of corticosteroids below 7.5 mg/day. The use of subcutaneous IFN-alpha 2a showed good results in all the eyes with resolution of inflammatory signs (vitritis and vasculitis), decrease in retinal thickening, and improvement in visual acuity without any major complications [96]. IFN-alpha 2a has also been used by Oray et al. in 5 patients with presumed IOTB for recurrent cystoid macular edema after completion of ATT. IFN-alpha 2a were shown to be safe and effective in managing macular edema in this series [97].
The challenge in the management of intraocular tuberculosis
Till date, there are no uniform guidelines in the management of IOTB. There are several regional variations in treatment practices for IOTB including the regime, duration of ATT, concomitant use of corticosteroids and immunosuppressive agents (both systemic and local). The treatment in a majority of IOTB cases is directed in consultation with the attending pulmonologist/internist based on guidelines that vary depending on the region of their practice. In the COTS-1 [25], analyses revealed variation in outcomes between different ethnic groups and geographical regions. For instance, the proportion of patients that received treatment with both ATT and corticosteroids was highly variable between Asia (80.4%), Australia (60.0%), and the West (62.9%). Thus, Asian patients are most likely to receive a combination of ATT and steroids compared to the Western countries. In addition, treatment outcomes on survival analysis were superior in patients of Asian ethnicity.
Dengue fever-associated uveitis
Dengue fever is a mosquito-borne viral illness caused by a flavivirus which has four serotypes. The most common arthropod vector for this condition is Aedes aegypti, which is common in tropical and subtropical regions. The mortality and morbidity due to dengue fever has tremendously increased in the tropical and sub-tropical zones of the world. Dengue fever can present with various vitreoretinal manifestations, where inflammation and ischemia are the hallmark features of the disease.
Epidemiology of dengue fever and recent outbreaks in Asia
Dengue is endemic world over in areas such as United States, Southeast Asia, and the Western Pacific. This arthropod borne disease is endemic in more than 100 countries, and is regarded as the most common mosquito-borne disease in humans [98,99,100]. The first case of dengue fever-related posterior uveitis was in 1979 amongst tourists who returned from dengue-endemic countries. More than 50 million dengue infections are estimated to occur annually throughout the world. Since the beginning of the 21st century, most cases have been reported from the South East Asian countries. There are four serotypes of the dengue virus. In countries such as Singapore, the number of cases have been steadily rising over the last few years [101]. Dengue and related uveitis have been reported from Singapore, Sri Lanka, Thailand, Taiwan, India, Mexico, and Brazil [102,103,104,105,106,107]. In Malaysia, a total of 101,357 dengue cases and 237 deaths were reported in 2016 [108]. Frequent outbreaks are also reported from Taiwan. From 2007 to 2011, a total of 3,322 confirmed dengue cases were noted in Taiwan, particularly from Kaohsiung city [109]. In 2017, the data from the National Vector Borne Disease Control Program (NVBDCP), India showed the highest number of cases and deaths due to dengue fever [110].
Chorioretinal manifestations of dengue fever
Ocular involvement in dengue may be unilateral or bilateral. Anterior segment features of dengue infection include subconjunctival hemorrhage, keratitis, anterior uveitis, and angle closure glaucoma. Most common posterior segment manifestations of dengue fever include macular edema, hemorrhages, foveolitis, cotton wool spots, and microaneurysms [111,112,113].
1. Dengue maculopathy: Dengue maculopathy is a common posterior segment condition and its incidence may correlate with the severity of systemic disease. Patients with dengue maculopathy may complain of visual symptoms such as blurring or scotomas due to outer retinal involvement [114, 115]. Fundus examination of this condition reveals arteriolar sheathing, cotton-wool spots, microaneurysms, intraretinal cystoid spaces, and macular edema (Fig. 4), perifoveal telangiectasia and intraretinal hemorrhages. There may be presence of well-defined yellowish subretinal lesions in the macular along with retinal striae radiating around the fovea (foveolitis). These lesions may represent disruption of photoreceptors (Fig. 5) [116,117,118,119,120].
Fundus photograph of a 23-year-old male who presented with low vision in the left eye 15 days after a severe febrile illness is shown (a). The patient was diagnosed with dengue fever based on NS1 antigen test positivity. The platelet nadir was 20,000/mm3. There was an altered foveal reflex with whitish retinal opacification along with retinal edema and hemorrhages. Optical coherence tomography B-scan shows presence of serous subretinal fluid, retinal thickening, and intraretinal cystoid spaces suggestive of dengue maculopathy (b)
Multimodal imaging of a patient (32-year-old female) with dengue maculopathy is shown here. The patient complained of scotoma in front of both her eyes 2 weeks after subsidence of dengue fever (tested positive for NS1 antigen). Fundus photograph revealed mild optic nerve hyperemia, and an altered red foveal reflex with yellow spots in the nasal macula (a). Optical coherence tomography B-scan (b) shows no evidence of retinal thickening; however, careful examination (c) revealed hyper-reflective spots in the outer nuclear layer (arrowheads). The deep retinal capillary plexus on optical coherence tomography angiography (d) showed evidence of capillary flow deficit suggestive of retinal vascular micro-occlusion
Acute macular neuroretinopathy: It is notable that acute macular neuroretinopathy (AMN) has been recently reported to be an unusual manifestation of dengue maculopathy [121, 122]. AMN presents with hyper-reflectivity of the outer retina (outer plexiform layer and outer nuclear layer), and disruption of ellipsoid zone, external limiting membrane (ELM) and inter-digitation zone.
2. Dengue vasculopathy: Dengue-related uveitis has been reported to present with retinal vasculitis. Vasculitis may be associated with retinal ischemia, intraretinal hemorrhages, and other manifestations such as branch retinal arteriolar occlusion. Capillary endothelial dysfunction or occlusion of precapillary arterioles due to immune complex deposition may be the likely underlying mechanism of retinal vasculitis in dengue fever [112, 113, 120].
3. Dengue chorioretinitis: One of the possible manifestations of dengue posterior uveitis includes chorioretinal involvement presenting with severe vitreous inflammation and exudative retinal detachment. Chorioretinitis may present with single or multiple multifocal lesions involving the macula along with retinal pigment epithelium disturbances. Chorioretinitis may evolve into atrophic perifoveal pigmentary scars (nummular scars). There may be associated choroidal thickening and evidence of neuroretinitis or papillitis [118, 119].
Imaging features of dengue-related uveitis: FA is a useful imaging modality to determine the extent and severity of retinal manifestations such as maculopathy and retinal vasculitis. Foveolitis appears as retinal pigment epithelial hyperfluorescence that appears in the early phase and persists till the late phase. There may be presence of macular periphlebitis and occlusion. Common findings include arteriolar leakage, macular edema, and disc leakage. ICGA may show presence of hypofluorescent spots suggestive of involvement of choriocapillaris and the retinal pigment epithelium [122,123,124].
OCT imaging is useful to diagnose presence of macular edema as well as other changes such as foveolitis. The OCT classification consisting of three patterns of dengue maculopathy as proposed by Teoh et al. [125] is as follows:
Type 1: diffuse retinal thickening: Type 1 maculopathy included patients with diffuse retinal edema with an increase in central and paracentral retinal thickness and loss of the normal foveal dimple. Patients with type 1 maculopathy were described to carry the best visual prognosis.
Type 2: cystoid macular edema: Type 2 maculopathy was characterized by large intraretinal cystoid spaces extending through the level of photoreceptors with reflective septae separating the cystoid cavities.
Type 3: foveolitis: Type 3 maculopathy was characterized by an area of thickening and high reflectivity in the outer retina at the foveal region. There may or may not be associated retinal edema.
The most common feature of OCT in dengue maculopathy is diffuse retinal thickening. Disruption of external limiting membrane, ellipsoid zone, and inter-digitation zone may also be noted in these patients [120, 125,126,127].
Dengue-induced inflammatory, ischemic foveolitis and outer maculopathy (DIII-FOM): In a recent outbreak of dengue virus fever in North India (2017), a number of patients presented with a unique set of posterior segment features such as mild posterior vitritis, disruption of outer retinal layers, conical retinal elevations, and retinal capillary flow deficits on OCT angiography. In these patients, both ischemia and inflammation appear to be the central mechanisms of visual loss due to dengue fever. Therefore, based on their clinical phenotype and fundus imaging characteristics, we have coined a new term for this entity, DIII-FOM (Fig. 6).
A patient with dengue-induced ischemic inflammatory foveolitis and outer maculopathy (DIII-FOM) shows retinal hemorrhages in the macula with greyish white parafoveal lesions (a). Autofluorescence imaging shows hypo-autofluorescence in the macular region (b). The optical coherence tomography (OCT) scan shows vitreous cells (white arrows), hyper-reflectivity of the outer plexiform (OPL) and outer nuclear layers, and hyper-reflective “conical foveal elevation” involving all retinal layers (black arrowhead) (c). There are cystoid spaces in the left eye (yellow arrow). OCT angiography shows an area of flow deficit (white arrow) in the perifoveal region in the superficial (d) and deep plexus (e)
Management of dengue-associated uveitis
There are no available guidelines regarding management of dengue-related maculopathy. Dengue maculopathy may be self-limiting in nature. In the presence of significant inflammation, topical, periocular, and systemic steroids may be used. Patients with AMN associated with dengue fever may also benefit from initiation of corticosteroids. However, there are no prospective trials assessing the efficacy of therapy till date. Thus, there is no clear evidence supporting the role of either steroids or intravenous immunoglobulin for the treatment of dengue retinochoroiditis or vasculitis [116, 120].
Chikungunya-associated uveitis
Chikungunya fever is a common arthropod-borne viral illness that commonly affects Asian countries and Pacific islands. Epidemics of chikungunya have been reported from several Asian countries such as India in the recent past. Chikungunya is caused by an Alphavirus which belongs to the family Togaviridae, a single-stranded RNA virus. The arthropod vector for chikungunya is the Aedes aegypti mosquito.
Epidemiology of chikungunya
Chikungunya fever can affect all ages and both sexes equally. This condition is endemic in Asia and Africa. The chikungunya virus was first isolated in Tanzania in 1953. More than 266,000 people were infected during the 2007 outbreak in Réunion and 1,400,000 cases were reported in India in 2006 [128]. Several outbreaks of chikungunya have been reported between 1957 and 1974. The disease is also endemic in Seychelles, Madagascar, Comoro Islands, Mauritius, and Mayotte. Other countries that are affected include Sri Lanka, Maldives, Malaysia and Indonesia [129,130,131,132]. Viral mutations may lead to newer genotypes of the Chikungunya virus (such as A226V) which have increased virulence and infectivity. Due to the lack of herd immunity and increased travel/globalization, even visitors who hail from Europe, Canada, United States, and Australia are susceptible to chikungunya fever [129, 133].
Chikungunya virus-associated uveitis
Chikungunya fever may have ocular manifestations which may be unilateral or bilateral. Ocular inflammation associated with chikungunya may present with symptoms such as redness, pain, diplopia, and retro-orbital heaviness. Anterior uveitis is the most common feature of this condition. There may be concomitant corneal involvement with dendritic pattern of keratic precipitates. Other features include raised intraocular pressure, episcleritis, and lagophthalmos [111, 134, 135].
Posterior segment manifestations of chikungunya infection include choroiditis, retinitis, optic neuritis, neuroretinitis, and panuveitis. The most common posterior segment features are retinitis with surrounding retinal edema and opacification. Retinal lesions may be associated with mild vitritis and disc edema. Severe inflammation may result in exudative retinal detachment, retinal vasculitis, and intraretinal hemorrhages. Published reports suggest that long-term implications of chikungunya-related posterior segment manifestations are poorly understood [117, 136,137,138,139].
Imaging features of chikungunya-related uveitis: Imaging tools such as FA and OCT are very useful in the evaluation and management of patients with chikungunya uveitis. FA reveals presence of early hypofluorescence followed by late hyperfluorescence corresponding to the area of retinitis. Similar to choroiditis associated with other etiologies, Chikungunya choroiditis shows early hypofluorescence followed by late leakage of dye on FA. On OCT, macular edema and retinal thickening may be observed (Fig. 7). OCT may also reveal presence of AMN in patients with chikungunya [135,136,137,138]. Features of AMN include hyper-reflectivity of the outer plexiform layer, outer nuclear layer, and disruption of ellipsoid zone, ELM and inter-digitation zone [140].
Fluorescein angiography (FA) and optical coherence tomography (OCT) of a 34-year-old male with retinitis. The patient tested IgM positive for chikungunya virus infection. FA imaging in the early phase shows hypofluorescence (a) followed by intense leakage in the late phase (b) suggestive of retinitis. OCT scan at presentation (c) shows retinal edema, intraretinal fluid, serous retinal detachment, and disruption of retinal layers. The patient was treated with oral corticosteroids and he also received an injection of posterior subtenon triamcinolone acetonide. At 3 months, there was significant resolution of retinal edema and serous retinal detachment (d). At 7-month follow-up, retinal thickening and retinitis lesions have resolved, and a thick epiretinal membrane is seen on OCT (e). (Image courtesy: Dr. Ankush Kawali, MS, Consultant, Uveitis Department, Narayana Nethralaya, Bangalore, India)
Management of chikungunya-related uveitis
There is no specific anti-viral therapy against Chikungunya virus. Therefore, the treatment of chikungunya is largely symptomatic including management of fever, intravenous fluid therapy, and symptomatic pain relief. Ocular inflammation can be treated with topical steroids and cycloplegic agents. Majority of patients with chikungunya posterior uveitis recover well with good visual outcome. In the presence of significant inflammation, including manifestations such as vision-threatening retinitis and AMN, systemic corticosteroids can be initiated to control the tissue damage [135, 136, 140].
Summary and conclusions
In the Asia Pacific region, TB, dengue and Chikungunya remain the major infections that continue to remain endemic and affect a large number of individuals. All the three entities are associated with potentially severe vision threatening uveitis. Entities such as TB may have protean clinical manifestations and may present with diagnostic challenges, especially because they can cause myriad conditions, including choroiditis, vasculitis, panuveitis, optic neuritis, endophthalmitis, and scleritis, among others. For establishing a proper diagnosis of IOTB, it is necessary to consider various factors such as endemicity, geographical location, ethnicity, and immigration status, as well as clinical and imaging features. Similarly, dengue and Chikungunya present with retinitis and/or vasculitis of varying severity. While there is no definitive therapy for these viral entities, supportive therapy corticosteroids may help to reduce the inflammatory damage. In summary, with the recent increase in the outbreaks and epidemics of dengue and Chikungunya fever, it is important to recognize the potentially vision-threatening posterior segment manifestations of these conditions.
References
Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23:705–17.
Majumder PD, Ghosh A, Biswas J. Infectious uveitis: an enigma. Middle East Afr J Ophthalmol. 2017;24:2–10.
Rathinam SR, Cunningham ET. Infectious causes of uveitis in the developing world. Int Ophthalmol Clin. 2000;40:137–52.
Gupta V, Shoughy SS, Mahajan S, Khairallah M, Rosenbaum JT, Curi A, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. 2015;23:14–24.
Chakraborty AK. Epidemiology of tuberculosis: current status in India. Indian J Med Res. 2004;120:248–76.
Chadha VK. Tuberculosis epidemiology in India: a review. Int J Tuberc Lung Dis. 2005;9:1072–82.
GBD Tuberculosis Collaborators. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18:261–84.
Vongpunsawad S, Intharasongkroh D, Thongmee T, Poovorawan Y. Seroprevalence of antibodies to dengue and chikungunya viruses in Thailand. PLoS ONE. 2017;12:e0180560.
Ang LW, Kam YW, Lin C, Krishnan PU, Tay J, Ng LC, et al. Seroprevalence of antibodies against chikungunya virus in Singapore resident adult population. PLoS Negl Trop Dis. 2017;11:e0006163.
Rodríguez-Barraquer I, Solomon SS, Kuganantham P, Srikrishnan AK, Vasudevan CK, Iqbal SH, et al. The hidden burden of dengue and chikungunya in Chennai, India. PLoS Negl Trop Dis. 2015;9:e0003906.
Capeding MR, Chua MN, Hadinegoro SR, IIHM Hussain, Nallusamy R, Pitisuttithum P, et al. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLoS Negl Trop Dis. 2013;7:e2331.
World Health Organization. Tuberculosis in the WHO South-East Asia region. Available at: http://www.who.int/bulletin/volumes/88/3/09-073874/en/ [Accessed 6 August 2018].
World Health Organization. Tuberculosis in India. Available at: http://www.who.int/countries/ind/en/ [Accessed 6 August 2018].
Das D, Biswas J, Ganesh SK. Pattern of uveitis in a referral uveitis clinic in India. Indian J Ophthalmol. 1995;43:117–21.
Singh R, Gupta V, Gupta A. Pattern of uveitis in a referral eye clinic in north India. Indian J Ophthalmol. 2004;52:121–5.
Dogra M, Singh R, Agarwal A, Sharma A, Singh SR, Gautam N, et al. Epidemiology of Uveitis in a Tertiary-care Referral Institute in North India. Ocul Immunol Inflamm. 2017;25(sup1):S46–S53.
Sabhapandit S, Murthy SI, Singh VM, Gaitonde K, Gopal M, Marsonia K, et al. Epidemiology and clinical features of uveitis from urban populations in South India. Ocul Immunol Inflamm. 2017;25(sup1):S39–S45.
Siak J, Kumaradas M, Chee S-P. The pattern of uveitis in Sri Lanka. Ocul Immunol Inflamm. 2017;25(sup1):S63–S68.
Nguyen M, Siak J, Chee S-P, Diem VQH. The Spectrum of Uveitis in Southern Vietnam. Ocul Immunol Inflamm. 2017;25(sup1):S100–S106.
Siak J, Jansen A, Waduthantri S, Teoh C-S, Jap A, Chee S-P. The pattern of uveitis among Chinese, Malays, and Indians in Singapore. Ocul Immunol Inflamm. 2017;25(sup1):S81–S93.
Gautam N, Singh R, Agarwal A, Yangzes S, Dogra M, Sharma A, et al. Pattern of pediatric uveitis at a Tertiary Referral Institute in North India. Ocul Immunol Inflamm. 2018;26:379–85.
Annamalai R, Biswas J. Patterns of intermediate uveitis in children presenting at a Tertiary Eye Care Center in South India. Middle East Afr J Ophthalmol. 2017;24:94–99.
Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol. 2007;52:561–87.
Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010;149:562–70.
Agrawal R, Gunasekeran DV, Grant R, Agarwal A, Kon OM, Nguyen QD, et al. Clinical features and outcomes of patients with tubercular uveitis treated with antitubercular therapy in the Collaborative Ocular Tuberculosis Study (COTS)-1. JAMA Ophthalmol. 2017;135:1318–27.
Gunasekeran DV, Agrawal R, Agarwal A, Carreño E, Raje D, Aggarwal K, et al. THE COLLABORATIVE OCULAR TUBERCULOSIS STUDY (COTS)-1: a multinational review of 251 patients with tubercular retinal vasculitis. Retina. 2018. https://doi.org/10.1097/IAE.0000000000002194.
Biswas J, Shome D. Choroidal tubercles in disseminated tuberculosis diagnosed by the polymerase chain reaction of aqueous humor. A case report and review of the literature. Ocul Immunol Inflamm. 2002;10:293–8.
Mehta S. Healing patterns of choroidal tubercles after antitubercular therapy: a photographic and OCT study. J Ophthalmic Inflamm Infect. 2012;2:95–97.
Gupta V, Gupta A, Sachdeva N, Arora S, Bambery P. Successful management of tubercular subretinal granulomas. Ocul Immunol Inflamm. 2006;14:35–40.
Agarwal M, Maitray A, Khetan V. Unusual presentation of choroidal tuberculoma. Ophthalmology. 2017;124:708.
Arej N, Fadlallah A, Chelala E. Choroidal tuberculoma as a presenting sign of tuberculosis. Int Med Case Rep J. 2016;9:365–8.
Alaraj AM, Al-Dhibi H, Al-Mezaine HS, Abu El-Asrar AM. Solitary presumed choroidal tuberculomas masquerading as choroidal tumors. Saudi Med J. 2013;34:86–90.
Mehta S. Fundus fluorescein angiography of choroidal tubercles: case reports and review of literature. Indian J Ophthalmol. 2006;54:273–5.
Invernizzi A, Agarwal A, Cozzi M, Viola F, Nguyen QD, Staurenghi G. Enhanced depth imaging optical coherence tomography features in areas of choriocapillaris hypoperfusion. Retina. 2016;36:2013–21.
Invernizzi A, Agarwal A, Mapelli C, Nguyen QD, Staurenghi G, Viola F. Longitudinal follow-up of choroidal granulomas using enhanced depth imaging optical coherence tomography. Retina. 2017;37:144–53.
Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119:2334–42.
Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152:857–63.e2.
Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginouslike choroiditis: clinical presentations and management. Ophthalmology. 2003;110:1744–9.
Varma D, Anand S, Reddy AR, Das A, Watson JP, Currie DC, et al. Tuberculosis: an under-diagnosed aetiological agent in uveitis with an effective treatment. Eye (Lond). 2006;20:1068–73.
Guedes ME, Galveia JN, Almeida AC, Costa JM. Tubercular serpiginous-like choroiditis. BMJ Case Rep. 2011 pii: bcr0820114654; 2011. https://doi.org/10.1136/bcr.08.2011.4654.
Znaor L, Medic A, Karaman K, Perkovic D. Serpiginous-like choroiditis as sign of intraocular tuberculosis. Med Sci Monit. 2011;17:CS88–90.
Kawali A, Emerson GG, Naik NK, Sharma K, Mahendradas P, Rao NA. Clinicopathologic features of tuberculous serpiginous-like choroiditis. JAMA Ophthalmol. 2018;136:219–21.
Bansal R, Basu S, Gupta A, Rao N, Invernizzi A, Kramer M. Imaging in tuberculosis-associated uveitis. Indian J Ophthalmol. 2017;65:264–70.
De Luigi G, Mantovani A, Papadia M, Herbort CP. Tuberculosis-related choriocapillaritis (multifocal-serpiginous choroiditis): follow-up and precise monitoring of therapy by indocyanine green angiography. Int Ophthalmol. 2012;32:55–60.
Agarwal A, Mahajan S, Khairallah M, Mahendradas P, Gupta A, Gupta V. Multimodal imaging in ocular tuberculosis. Ocul Immunol Inflamm. 2017;25:134–45.
Nazari Khanamiri H, Rao NA. Serpiginous choroiditis and infectious multifocal serpiginoid choroiditis. Surv Ophthalmol. 2013;58:203–32.
Gupta A, Bansal R, Gupta V, Sharma A. Fundus autofluorescence in serpiginouslike choroiditis. Retina. 2012;32:814–25.
Bansal R, Kulkarni P, Gupta A, Gupta V, Dogra MR. High-resolution spectral domain optical coherence tomography and fundus autofluorescence correlation in tubercular serpiginouslike choroiditis. J Ophthalmic Inflamm Infect. 2011;1:157–63.
Gupta A, Biswas J. Fundus autofluorescence imaging to document evolution, progression and healing pattern of serpiginous choroiditis. Oman J Ophthalmol. 2014;7:100–1.
Cardillo Piccolino F, Grosso A, Savini E. Fundus autofluorescence in serpiginous choroiditis. Graefes Arch Clin Exp Ophthalmol. 2009;247:179–85.
Carreño E, Portero A, Herreras JM, López MI. Assesment of fundus autofluorescence in serpiginous and serpiginous-like choroidopathy. Eye. 2012;26:1232–6.
Aggarwal K, Mulkutkar S, Mahajan S, Singh R, Sharma A, Bansal R, et al. Role of ultra-wide field imaging in the management of tubercular posterior uveitis. Ocul Immunol Inflamm. 2016;24:631–6.
Campbell JP, Leder HA, Sepah YJ, Gan T, Dunn JP, Hatef E, et al. Wide-field retinal imaging in the management of noninfectious posterior uveitis. Am J Ophthalmol. 2012;154:908–11.e2.
Dickson D, Agarwal A, Sadiq MA, Hassan M, High R, Nguyen QD, et al. Assessment of vitreous haze using ultra-wide field retinal imaging. J Ophthalmic Inflamm Infect. 2016;6:35.
Gupta V, Al-Dhibi HA, Arevalo JF. Retinal imaging in uveitis. Saudi J Ophthalmol. 2014;28:95–103.
Rifkin LM, Munk MR, Baddar D, Goldstein DA. A new OCT finding in tuberculous serpiginous-like choroidopathy. Ocul Immunol Inflamm. 2015;23:53–58.
Mandadi SKR, Agarwal A, Aggarwal K, Moharana B, Singh R, Sharma A, et al. Novel findings on optical coherence tomography angiography in patients with tubercular serpiginous-like choroiditis. Retina. 2017;37:1647–59.
Aggarwal K, Agarwal A, Sharma A, Sharma K, Gupta V, OCTA Study Group. Detection of type 1 choroidal neovascular membranes using optical coherence tomography angiography in tubercular posterior uveitis. Retina. 2018.
Agarwal M, Shrivastav A, Waris A. Tubercular retinal vasculitis mimicking frosted branch angiitis: a case report. J Ophthalmic Inflamm Infect. 2018;8:3.
Agrawal R, Gunasekeran DV, Gonzalez-Lopez JJ, Cardoso J, Gupta B, Addison PKF, et al. PERIPHERAL RETINAL VASCULITIS: analysis of 110 consecutive cases and a contemporary reappraisal of tubercular etiology. Retina. 2017;37:112–7.
Biswas J, Ravi RK, Naryanasamy A, Kulandai LT, Madhavan HN. Eales’ disease - current concepts in diagnosis and management. J Ophthalmic Inflamm Infect. 2013;3:11.
Biswas J, KRR, Pal B, Gondhale HP, Kharel Sitaula R. Long-term outcomes of a large cohort of patients with Eales’ disease. Ocul Immunol Inflamm. 2018;26:870–6.
Therese KL, Deepa P, Therese J, Bagyalakshmi R, Biswas J, Madhavan HN. Association of mycobacteria with Eales’ disease. Indian J Med Res. 2007;126:56–62.
Verma A, Biswas J, Dhanurekha L, Gayathri R, Lily Therese K. Detection of Mycobacterium tuberculosis with nested polymerase chain reaction analysis in enucleated eye ball in Eales’ disease. Int Ophthalmol. 2016;36:413–7.
Singh R, Toor P, Parchand S, Sharma K, Gupta V, Gupta A. Quantitative polymerase chain reaction for Mycobacterium tuberculosis in so-called Eales’ disease. Ocul Immunol Inflamm. 2012;20:153–7.
Agarwal A, Karkhur S, Aggarwal K, Invernizzi A, Singh R, Dogra MR, et al. Epidemiology and clinical features of inflammatory retinal vascular occlusions: pooled data from two tertiary-referral institutions. Clin Exp Ophthalmol. 2018;46:62–74.
Agarwal A, Afridi R, Agrawal R, Do DV, Gupta V, Nguyen QD. Multimodal imaging in retinal vasculitis. Ocul Immunol Inflamm. 2017;25:424–33.
Nayak S, Basu S, Singh MK. Presumed tubercular retinal vasculitis with serpiginous-like choroiditis in the other eye. Ocul Immunol Inflamm. 2011;19:361–2.
Gupta P, Singh R, Gupta S, Kumar A, Kakkar N. Tuberculosis presenting as posttraumatic panophthalmitis. Oman J Ophthalmol. 2016;9:52–54.
Demirci H, Shields CL, Shields JA, Eagle RC. Ocular tuberculosis masquerading as ocular tumors. Surv Ophthalmol. 2004;49:78–89.
Takkar A, Mahesh KV, Shree R, Sachdeva J, Mehta S, Lal V. Tuberculomas in ‘critical’ locations of the visual pathway-a Masquerader. Neuroophthalmology . 2018;42:109–11.
Padhi TR, Basu S, Das T, Samal B. Optic disc tuberculoma in a patient with miliary tuberculosis. Ocul Immunol Inflamm. 2011;19:67–68.
Sivakumar P, Vedachalam R, Devy N. Management challenge: optic disc granuloma in pulmonary tuberculosis. Indian J Ophthalmol. 2018;66:301.
Aggarwal K, Agarwal A, Sehgal S, Sharma S, Singh N, Sharma K, et al. An unusual presentation of intraocular tuberculosis in a monocular patient: clinicopathological correlation. J Ophthalmic Inflamm Infect. 2016;6:46.
Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23:7–13.
Bansal R, Gupta A, Gupta V, Dogra MR, Bambery P, Arora SK. Role of anti-tubercular therapy in uveitis with latent/manifest tuberculosis. Am J Ophthalmol. 2008;146:772–9.
Agrawal R, Gupta B, Gonzalez-Lopez JJ, Rahman F, Phatak S, Triantafyllopoulou I, et al. The role of anti-tubercular therapy in patients with presumed ocular tuberculosis. Ocul Immunol Inflamm. 2015;23:40–46.
Kee AR, Gonzalez-Lopez JJ, Al-Hity A, Gupta B, Lee CS, Gunasekeran DV, et al. Anti-tubercular therapy for intraocular tuberculosis: a systematic review and meta-analysis. Surv Ophthalmol. 2016;61:628–53.
Ang M, Hedayatfar A, Wong W, Chee S-P. Duration of anti-tubercular therapy in uveitis associated with latent tuberculosis: a case-control study. Br J Ophthalmol. 2012;96:332–6.
Alvarez GG, Roth VR, Hodge W. Ocular tuberculosis: diagnostic and treatment challenges. Int J Infect Dis. 2009;13:432–5.
Souissi S, David T, Beral L. Steroid treatment in ocular tuberculosis: a double-edged sword? J Fr Ophtalmol. 2017;40:126–32.
Basu S, Das T. Pitfalls in the management of TB-associated uveitis. Eye. 2010;24:1681–4.
Tomkins-Netzer O, Leong BCS, Zhang X, Lightman S, McCluskey PJ.Sydney-London Latent Ocular TB Study Group. Effect of antituberculous therapy on uveitis associated with latent tuberculosis. Am J Ophthalmol. 2018;190:164–70.
Shakarchi FI. Ocular tuberculosis: current perspectives. Clin Ophthalmol. 2015;9:2223–7.
Esen E, Sızmaz S, Kunt Z, Demircan N. Paradoxical worsening of tubercular serpiginous-like choroiditis after initiation of antitubercular therapy. Turk J Ophthalmol. 2016;46:186–9.
Ganesh SK, Ali BS. Paradoxical worsening of a case of TB subretinal abscess with serpiginous-like choroiditis following the initiation of antitubercular therapy. Indian J Ophthalmol. 2017;65:761–4.
Cheung CMG, Chee SP. Jarisch-Herxheimer reaction: paradoxical worsening of tuberculosis chorioretinitis following initiation of antituberculous therapy. Eye. 2009;23:1472–3.
Agarwal A, Aggarwal K, Deokar A, Mandadi SKR, Singh SR, Singh R, et al. Optical coherence tomography angiography features of paradoxical worsening in tubercular multifocal serpiginoid choroiditis. Ocul Immunol Inflamm. 2016;24:621–30.
Aggarwal K, Agarwal A, Deokar A, Singh R, Bansal R, Sharma A, et al. Ultra-wide field imaging in paradoxical worsening of tubercular multifocal serpiginoid choroiditis after the initiation of anti-tubercular therapy. Ocul Immunol Inflamm. 2017:1–6. https://doi.org/10.1080/09273948.2017.1373829
Fonollosa A, Valsero S, Artaraz J, Ruiz-Arruza I. Dexamethasone intravitreal implants in the management of tubercular multifocal serpiginoid choroiditis. J Ophthalmic Inflamm Infect. 2016;6:31.
Jain L, Panda KG, Basu S. Clinical outcomes of adjunctive sustained-release intravitreal dexamethasone implants in tuberculosis-associated multifocal serpigenoid choroiditis. Ocul Immunol Inflamm. 2018;26:877–83.
Agarwal A, Handa S, Aggarwal K, Sharma M, Singh R, Sharma A, et al. The role of dexamethasone implant in the management of tubercular uveitis. Ocul Immunol Inflamm. 2018;26:884–92.
Julian K, Langner-Wegscheider B-J, Haas A, De Smet MD. Intravitreal methotrexate in the management of presumed tuberculous serpiginous-like choroiditis. Retina. 2013;33:1943–8.
Sahin O, Ziaei A. The role of methotrexate in resolving ocular inflammation after specific therapy for presumed latent syphilitic uveitis and presumed tuberculosis-related uveitis. Retina. 2014;34:1451–9.
La Distia Nora R, Walburg KV, van Hagen PM, Swagemakers SMA, van der Spek PJ, Quinten E, et al. Retinal pigment epithelial cells control early Mycobacterium tuberculosis infection via interferon signaling. Invest Ophthalmol Vis Sci. 2018;59:1384–95.
Invernizzi A, Iannaccone F, Marchi S, Mastrofilippo V, Coassin M, Fontana L, et al. Interferon alpha-2a for the treatment of post-infectious uveitis secondary to presumed intraocular tuberculosis. Ocul Immunol Inflamm. 2018:1–8. https://doi.org/10.1080/09273948.2018.1431292
Oray M, Onal S, Uludag G, Akbay AK, Tugal-Tutkun I. Interferon alpha for the treatment of cystoid macular edema associated with presumed ocular tuberculosis. J Ocul Pharmacol Ther. 2017;33:304–12.
Gyawali N, Bradbury RS, Taylor-Robinson AW. The epidemiology of dengue infection: harnessing past experience and current knowledge to support implementation of future control strategies. J Vector Borne Dis. 2016;53:293–304.
Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2017; 7. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5506197/ [Accessed 3 June 2018].
Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309.
Lee K-S, Lo S, Tan SS-Y, Chua R, Tan L-K, Xu H, et al. Dengue virus surveillance in Singapore reveals high viral diversity through multiple introductions and in situ evolution. Infect Genet Evol. 2012;12:77–85.
Chang JH, McCluskey PJ, Wakefield D. Acute anterior uveitis and HLA-B27. Surv Ophthalmol. 2005;50:364–88.
Prayitno A, Taurel A-F, Nealon J, Satari HI, Karyanti MR, Sekartini R, et al. Dengue seroprevalence and force of primary infection in a representative population of urban dwelling Indonesian children. PLoS Negl Trop Dis. 2017;11:e0005621.
Choudhary MC, Gupta E, Sharma S, Hasnain N, Agarwala P. Genetic signatures coupled with lineage shift characterise endemic evolution of Dengue virus serotype 2 during 2015 outbreak in Delhi, India. Trop Med Int Health. 2017;22:871–80.
Wartel TA, Prayitno A, Hadinegoro SRS, Capeding MR, Thisyakorn U, Tran NH, et al. Three decades of dengue surveillance in five highly endemic South East Asian countries. Asia Pac J Public Health. 2017;29:7–16.
Chen SP. Molecular evolution and epidemiology of four serotypes of dengue virus in Thailand from 1973 to 2007. Epidemiol Infect. 2013;141:419–24.
Uehara A, Tissera HA, Bodinayake CK, Amarasinghe A, Nagahawatte A, Tillekeratne LG, et al. Analysis of dengue serotype 4 in Sri Lanka during the 2012-2013 dengue epidemic. Am J Trop Med Hyg. 2017;97:130–6.
Ahmad R, Suzilah I, Wan Najdah WMA, Topek O, Mustafakamal I, Lee HL. Factors determining dengue outbreak in Malaysia. PLoS ONE. 2018;13:e0193326.
Chang C-J, Chen CS, Tien C-J, Lu M-R. Epidemiological, clinical and climatic characteristics of dengue fever in Kaohsiung City, Taiwan with implication for prevention and control. PLoS ONE. 2018;13:e0190637.
Anon. National Vector Borne Disease Control Programme. Dengue Cases and Deaths in the Country since 2014. Available at: http://nvbdcp.gov.in/den-cd.html [Accessed 3 June 2018].
Lee JH, Agarwal A, Mahendradas P, Lee CS, Gupta V, Pavesio CE, et al. Viral posterior uveitis. Surv Ophthalmol. 2017;62:404–45.
Siqueira RC, Vitral NP, Campos WR, Oréfice F, de Moraes, Figueiredo LT. Ocular manifestations in dengue fever. Ocul Immunol Inflamm. 2004;12:323–7.
Lim W-K, Mathur R, Koh A, Yeoh R, Chee S-P. Ocular manifestations of dengue fever. Ophthalmology. 2004;111:2057–64.
Fang PP, Pfau M, Holz FG, Finger RP. Persistent visual loss in dengue fever due to outer retinal damage. Clin Exp Ophthalmol. 2017;45:747–9.
Nah G, Tan M, Teoh S, Chong CH. Maculopathy associated with dengue fever in a military pilot. Aviat Space Environ Med. 2007;78:1064–7.
Chan DPL, Teoh SCB, Tan CSH, Nah GKM, Rajagopalan R, Prabhakaragupta MK, et al. Ophthalmic complications of dengue. Emerg Infect Dis. 2006;12:285–9.
Khairallah M, Kahloun R, Ben Yahia S, Jelliti B, Messaoud R. New infectious etiologies for posterior uveitis. Ophthalmic Res. 2013;49:66–72.
Yadav HM, Dutta Majumder P, Biswas J. Dengue associated choroiditis: a rare entity. J Ophthalmic Inflamm Infect. 2017;7:14.
Tabbara K. Dengue retinochoroiditis. Ann Saudi Med. 2012;32:530–3.
Ng AW, Teoh SC. Dengue eye disease. Surv Ophthalmol. 2015;60:106–14.
Munk MR, Jampol LM, Cunha Souza E, de Andrade GC, Esmaili DD, Sarraf D, et al. New associations of classic acute macular neuroretinopathy. Br J Ophthalmol. 2016;100:389–94.
Aggarwal K, Agarwal A, Katoch D, Sharma M, Gupta V. Optical coherence tomography angiography features of acute macular neuroretinopathy in dengue fever. Indian J Ophthalmol. 2017;65:1235–8.
Juanarita J, Azmi MNR, Azhany Y, Liza-Sharmini AT. Dengue related maculopathy and foveolitis. Asian Pac J Trop Biomed. 2012;2:755–6.
Akanda M, Gangaputra S, Kodati S, Melamud A, Sen HN. Multimodal imaging in dengue-fever-associated maculopathy. Ocul Immunol Inflamm. 2018;26:671–6.
Teoh SC, Chee CK, Laude A, Goh KY, Barkham T, Ang BS, et al. Optical coherence tomography patterns as predictors of visual outcome in dengue-related maculopathy. Retina. 2010;30:390–8.
Mehkri M, Jayadev C, Dave N, Vinekar A. Spectral domain optical coherence tomography in the diagnosis and monitoring of dengue maculopathy. Indian J Ophthalmol. 2015;63:342–3.
Rhee TK, Han JI. Use of optical coherence tomography to evaluate visual acuity and visual field changes in dengue fever. Korean J Ophthalmol. 2014;28:96–99.
Javelle E, Ribera A, Degasne I, Gaüzère B-A, Marimoutou C, Simon F. Specific management of post-chikungunya rheumatic disorders: a retrospective study of 159 cases in Reunion Island from 2006-2012. PLoS Negl Trop Dis. 2015;9:e0003603.
Ngwe Tun MM, Inoue S, Thant KZ, Talemaitoga N, Aryati A, Dimaano EM, et al. Retrospective seroepidemiological study of chikungunya infection in South Asia, Southeast Asia and the Pacific region. Epidemiol Infect. 2016;144:2268–75.
Kalantri SP, Joshi R, Riley LW. Chikungunya epidemic: an Indian perspective. Natl Med J India. 2006;19:315–22.
Nagpal BN, Saxena R, Srivastava A, Singh N, Ghosh SK, Sharma SK, et al. Retrospective study of chikungunya outbreak in urban areas of India. Indian J Med Res. 2012;135:351–8.
Gasque P, Couderc T, Lecuit M, Roques P, Ng LFP. Chikungunya virus pathogenesis and immunity. Vector Borne Zoonotic Dis. 2015;15:241–9.
Pialoux G, Gaüzère B-A, Jauréguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–27.
Rocha VFD, de Oliveira AHP, Bandeira AC, Sardi SI, Garcia RF, Magalhães S et al. Chikungunya virus infection associated with encephalitis and anterior uveitis. Ocul Immunol Inflamm. 2018;26:677–9.
Mahendradas P, Ranganna SK, Shetty R, Balu R, Narayana KM, Babu RB, et al. Ocular manifestations associated with chikungunya. Ophthalmology. 2008;115:287–91.
Kawali A, Mahendradas P, Mohan A, Mallavarapu M, Shetty B Epidemic retinitis. Ocul Immunol Inflamm. 2018:1–7. https://doi.org/10.1080/09273948.2017.1421670.
Scripsema NK, Sharifi E, Samson CM, Kedhar S, Rosen RB. Chikungunya-associated uveitis and exudative retinal detachment: a case report. Retin Cases Brief Rep. 2015;9:352–6.
Martínez-Pulgarín DF, Chowdhury FR, Villamil-Gomez WE, Rodriguez-Morales AJ, Blohm GM, Paniz-Mondolfi AE. Ophthalmologic aspects of chikungunya infection. Travel Med Infect Dis. 2016;14:451–7.
Khairallah M, Kahloun R. Ocular manifestations of emerging infectious diseases. Curr Opin Ophthalmol. 2013;24:574–80.
Agarwal A, Choudhary T, Gupta V. Optical coherence tomography angiography features of bilateral retinopathy associated with Chikungunya fever. Indian J Ophthalmol. 2018;66:142–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Agarwal, A., Aggarwal, K. & Gupta, V. Infectious uveitis: an Asian perspective. Eye 33, 50–65 (2019). https://doi.org/10.1038/s41433-018-0224-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0224-y