Abstract
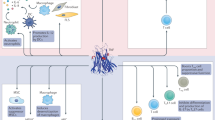
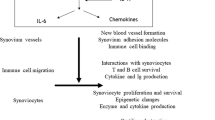
Despite their different targets, biologic agents used for blockade of TNF and IL-6, inhibition of T-cell co-stimulation and B-cell depletion all have similar beneficial effects on the outcome of rheumatoid arthritis (RA). This observation raises questions as to whether the targets of these therapies might all be involved in a common pathogenetic pathway. However, blockade of TNF and IL-6 has a similar inhibitory effect on joint damage progression in patients with either early or late disease. In comparison, B-cell depletion and inhibition of T-cell co-stimulation seem to have a somewhat delayed effect on joint damage (compared with cytokine inhibition), which suggests that these approaches affect upstream pathogenetic events. This article discusses these disparities and presents hypotheses as to whether clinical trial data can be used to determine at which point a biologic agent might interfere with the pathogenetic cascade in RA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feldmann, M., Brennan, F. M., Foxwell, B. M. & Maini, R. N. The role of TNF-α and IL-1 in rheumatoid arthritis. Curr. Dir. Autoimmun. 3, 188–199 (2001).
Partsch, G. et al. Highly increased levels of tumor necrosis factor-α and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J. Rheumatol. 24, 518–523 (1997).
Firestein, G. S., Alvaro-Gracia, J. M. & Maki, R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J. Immunol. 144, 3347–3353 (1990).
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010).
Smolen, J. S. & Steiner, G. Rheumatoid arthritis is more than cytokines: autoimmunity and rheumatoid arthritis. Arthritis Rheum. 44, 2218–2220 (2001).
Buch, M. H. et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 909–920 (2011).
Westhovens, R. et al. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann. Rheum. Dis. 68, 1870–1877 (2009).
Dinarello, C. A. Interleukin-1 and interleukin-1 antagonism. Blood 77, 1627–1652 (1991).
van Den Berg, W. B. Lessons from animal models of arthritis over the past decade. Arthritis Res. Ther. 11, 250 (2009).
Cohen, S. B. The use of anakinra, an interleukin-1 receptor antagonist, in the treatment of rheumatoid arthritis. Rheum. Dis. Clin. North Am. 30, 365–380 (2004).
Cardiel, M. H. et al. A phase 2 randomized, double-blind study of AMG 108, a fully human monoclonal antibody to IL-1R, in patients with rheumatoid arthritis. Arthritis Res. Ther. 12, R192 (2010).
Smolen, J. S. et al. The need for prognosticators in rheumatoid arthritis. Biological and clinical markers: where are we now? Arthritis Res. Ther. 10, 208 (2008).
van der Lubbe, P. A., Dijkmans, B. A., Markusse, H. M., Nässander, U. & Breedveld, F. C. A randomized, double-blind, placebo-controlled study of CD4 monoclonal antibody therapy in early rheumatoid arthritis. Arthritis Rheum. 38, 1097–1106 (1995).
van Vollenhoven, R. F., Kinnman, N., Vincent, E., Wax, S. & Bathon, J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. 63, 1782–1792 (2011).
Human Genome Sciences. Human genome sciences reports phase 2 results for lymphostat-b (belimumab) in patients with rheumatoid arthritis [online], (2005).
Keystone, E. Treatments no longer in development for rheumatoid arthritis. Ann. Rheum. Dis. 61 (Suppl. 2) ii43–ii45 (2002).
Genovese, M. C. et al. Combination therapy with etanercept and anakinra in the treatment of patients with rheumatoid arthritis who have been treated unsuccessfully with methotrexate. Arthritis Rheum. 50, 1412–1419 (2004).
Weinblatt, M. et al. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum. 54, 2807–2816 (2006).
Kleinau, S., Martinsson, P. & Heyman, B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcγ receptors. J. Exp. Med. 191, 1611–1616 (2000).
Alonzi, T. et al. Interleukin 6 is required for the development of collagen-induced arthritis. J. Exp. Med. 187, 461–468 (1998).
Gouze, E. et al. Gene therapy for rheumatoid arthritis. Expert Opin. Biol. Ther. 1, 971–978 (2001).
Blüml, S. et al. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 63, 1281–1288 (2011).
Smolen, J. S., Aletaha, D., Koeller, M., Weisman, M. H. & Emery, P. New therapies for the treatment of rheumatoid arthritis. Lancet 370, 1861–1874 (2007).
Hamel, K. et al. Suppression of proteoglycan-induced arthritis by anti-CD20 B cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J. Immunol. 180, 4994–5003 (2008).
Raza, K. et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res. Ther. 7, R784–R795 (2005).
Chen, X. & Oppenheim, J. J. Contrasting effects of TNF and anti-TNF on the activation of effector T cells and regulatory T cells in autoimmunity. FEBS Lett. 585, 3611–3618 (2011).
Yoshida, H., Hashizume, M., Suzuki, M. & Mihara, M. Anti-IL-6 receptor antibody suppressed T cell activation by inhibiting IL-2 production and inducing regulatory T cells. Eur. J. Pharmacol. 634, 178–183 (2010).
Dörner, T., Radbruch, A. & Burmester, G. R. B-cell-directed therapies for autoimmune disease. Nature Rev. Rheumatol. 5, 433–441 (2009).
Hamel, K. M. et al. B cell depletion enhances T regulatory cell activity essential in the suppression of arthritis. J. Immunol. 187, 4900–4906 (2011).
Sun, M. & Fink, P. J. A new class of reverse signaling costimulators belongs to the TNF family. J. Immunol. 179, 4307–4312 (2007).
Axmann, R. et al. CTLA-4 directly inhibits osteoclast formation. Ann. Rheum. Dis. 67, 1603–1609 (2008).
Álvarez-Quiroga, C. et al. CTLA-4-Ig therapy diminishes the frequency but enhances the function of TREG cells in patients with rheumatoid arthritis. J. Clin. Immunol. 31, 588–595 (2011).
Emery, P. et al. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 372, 375–382 (2008).
Keystone, E. et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 58, 3319–3329 (2008).
Emery, P. et al. The effects of golimumab on radiographic progression in rheumatoid arthritis: results of randomized controlled studies of golimumab before methotrexate therapy and golimumab after methotrexate therapy. Arthritis Rheum. 63, 1200–1210 (2011).
Smolen, J. S. et al. Evidence of radiographic benefit of infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of the ATTRACT trial. Arthritis Rheum. 52, 1020–1030 (2005).
Kremer, J. M. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 63, 609–621 (2011).
Smolen, J. S., Martinez-Avila, J. C. & Aletaha, D. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its antiinflammatory effects: disassociation of the link between inflammation and destruction. Ann. Rheum. Dis. http://dx.doi.org/10.1136/annrheumdis-2011-200395.
Tak, P. P. et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann. Rheum. Dis. 70, 39–46 (2011).
Aletaha, D., Alasti, F. & Smolen, J. S. Rheumatoid arthritis near remission: clinical rather than laboratory inflammation is associated with radiographic progression. Ann. Rheum. Dis. 70, 1975–1980 (2011).
Smolen, J. S., Aletaha, D., Grisar, J. C., Stamm, T. A. & Sharp, J. T. Estimation of a numerical value for joint damage-related physical disability in rheumatoid arthritis clinical trials. Ann. Rheum. Dis. 69, 1058–1064 (2010).
Dawes, P. T. et al. Rheumatoid arthritis: treatment which controls the C-reactive protein and erythrocyte sedimentation rate reduces radiological progression. Br. J. Rheumatol. 25, 44–49 (1986).
Elliott, M. J. et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet 344, 1105–1110 (1994).
Takagi, N. et al. Blockage of interleukin-6 receptor ameliorates joint disease in murine collagen-induced arthritis. Arthritis Rheum. 41, 2117–2121 (1998).
Williams, R. O., Feldmann, M. & Maini, R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl Acad. Sci. USA 89, 9784–9788 (1992).
Yanaba, K. et al. B cell depletion delays collagen-induced arthritis in mice: arthritis induction requires synergy between humoral and cell-mediated immunity. J. Immunol. 179, 1369–1380 (2007).
Knoerzer, D. B., Karr, R. W., Schwartz, B. D. & Mengle–Gaw, L. J. Collagen-induced arthritis in the BB rat. Prevention of disease by treatment with CTLA-4-Ig. J. Clin. Invest. 96, 987–993 (1995).
Iwai, H. et al. Amelioration of collagen-induced arthritis by blockade of inducible costimulator-B7 homologous protein costimulation. J. Immunol. 169, 4332–4339 (2002).
St. Clair, E. W. et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: a randomized, controlled trial. Arthritis Rheum. 50, 3432–3443 (2004).
Breedveld, F. C. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 54, 26–37 (2006).
Keystone, E. C. et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 50, 1400–1411 (2004).
Cohen, S. B. et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 54, 2739–2806 (2006).
Keystone, E. et al. Rituximab inhibits structural joint damage in patients with rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitor therapies. Ann. Rheum. Dis. 68, 216–221 (2009).
Lipsky, P. E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 343, 1594–1602 (2000).
Klareskog, L. et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363, 675–681 (2004).
Kremer, J. M. et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 144, 865–876 (2006).
Acknowledgements
The authors are supported in part through Coordination Theme 1 (Health) of the European Community's FP7; Grant Agreement number HEALTH-F2-2008-223404 (Masterswitch). This is a publication of the Joint and Bone Center for Diagnosis, Research and Therapy of Musculoskeletal Disorders of the Medical University of Vienna, Austria.
Author information
Authors and Affiliations
Contributions
All authors researched the data for, and wrote, the manuscript as well as providing substantial contributions to discussions of the content and editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
J. S. Smolen declares that he has acted as a speaker or consultant, and received research funding from Abbott, Amgen, Bristol-Myers Squibb, Janssen Biotech, MSD, Pfizer, Roche and UCB. D. Aletaha declares that he has acted as a speaker or consultant for Abbott, Bristol-Myers Squibb, MSD, Pfizer, Roche and UCB. K. Redlich declares that he has acted as a speaker or consultant for Abbott, MSD, Pfizer and Roche.
Rights and permissions
About this article
Cite this article
Smolen, J., Aletaha, D. & Redlich, K. The pathogenesis of rheumatoid arthritis: new insights from old clinical data?. Nat Rev Rheumatol 8, 235–243 (2012). https://doi.org/10.1038/nrrheum.2012.23
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2012.23