Abstract
The low incidence of idiosyncratic drug-induced liver injury (DILI), together with the lack of a reliable diagnostic biomarker and robust preclinical and in vitro toxicology test systems for the condition have limited our ability to define the mechanisms of DILI. A notable exception is acetaminophen hepatotoxicity, which is associated with the formation of a well-characterized and highly reactive intermediate metabolite, N-acetyl-p-benzoquinone imine. However, studies have also suggested a role for the host immune response and variation in the expression of the lymphocyte CD44 gene in the pathogenesis of acetaminophen hepatotoxicity. A careful review of the laboratory, clinical and histological phenotype of patients with DILI can provide potential clues to the mechanisms of disease pathogenesis, as observed with fialuridine and valproate hepatotoxicity. In addition, the use of transcriptomic and genomic approaches in patients with well-characterized DILI has provided important insights into the involvement of the host immune response in the pathogenesis of hepatotoxicity associated with the administration of flucloxacillin, lumiracoxib or ximelagatran. This Review highlights new developments regarding the potential role of reactive metabolites, mitochondrial toxicity, host immune-response pathways and biliary transporters in the etiopathogenesis of DILI. Going forward, a bedside-to-bench approach could improve our understanding of the mechanisms and risk factors for DILI.
Key Points
-
The biological basis for most instances of drug-induced liver injury (DILI) is unknown, but variation in host metabolic, detoxification, liver-regeneration and immune-response pathways has been implicated
-
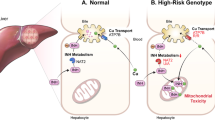
Results of studies in animal models and humans suggest a role for variation in the expression of CD44 in acetaminophen hepatotoxicity
-
Genomic approaches have demonstrated that variation in the host immune response could help to explain susceptibility to DILI induced by treatment with ximelagatran, lumiracoxib or flucloxacillin
-
Informative animal models of DILI and in vitro test systems to predict drug hypersensitivity reactions are currently lacking
-
A bedside-to-bench approach involving the collection of biological samples from patients with well-characterized DILI could improve our understanding of the risk factors and mechanisms of DILI
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ostapowicz, G. et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137, 947–954 (2002).
Sgro, C. et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 36, 451–455 (2002).
Chalasani, N. et al. Causes, clinical features and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 135, 1924–1934 (2008).
Temple, R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol. Drug Saf. 15, 241–243 (2006).
Larson, A. M. et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42, 1364–1372 (2005).
Fontana, R. J. et al. Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology 52, 730–742 (2010).
Agarwal, V. K., McHutchison, J. G. & Hoofnagle, J. H. Important elements for the diagnosis of drug-induced liver injury. Clin. Gastroenterol. Hepatol. 8, 463–470 (2010).
Watkins, P. B. et al. Aminotransferase elevations in healthy adults receiving 4 grams of acetaminophen daily: a randomized controlled trial. JAMA 296, 87–93 (2006).
Kuffner, E. K., Temple, A. R., Cooper, K. M., Baggish, J. S. & Parenti, D. L. Retrospective analysis of transient elevations in alanine aminotransferase during long-term treatment with acetaminophen in osteoarthritis clinical trials. Curr. Med. Res. Opin. 22, 2137–2148 (2006).
Nelson, S. D. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 10, 267–278 (1990).
Makin, A. J., Wendon, J. & Williams, R. A 7-year experience of severe acetaminophen-induced hepatotoxicity (1987–1993). Gastroenterology 109, 1907–1916 (1995).
Zimmerman, H. J. & Maddrey, W. C. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 22, 767–773 (1995).
Slattery, J. T., Nelson, S. D. & Thummel, K. E. The complex interaction between ethanol and acetaminophen. Clin. Pharmacol. Ther. 60, 241–246 (1996).
Mitchell, J. R. et al. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 187, 185–194 (1973).
Jollow, D. J. et al. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J. Pharmacol. Exp. Ther. 187, 195–202 (1973).
James, L. P. et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab. Dispos. 37, 1779–1784 (2009).
Davern, T. J. 2nd et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 130, 687–694 (2006).
Harrill, A. H. et al. Mouse population-guided resequencing reveals that variants in CD44 contribute to acetaminophen-induced liver injury in humans. Genome Res. 19, 1507–1515 (2009).
Kimura, K. et al. Critical role of CD44 in hepatotoxin-mediated liver injury. J. Hepatol. 48, 952–961 (2008).
Fannin, R. D. et al. Acetaminophen dosing of humans results in blood transcriptome and metabolome changes consistent with impaired oxidative phosphorylation. Hepatology 51, 227–236 (2010).
Kimura, K., Hayashi, S. & Nagaki, M. Roles of CD44 in chemical-induced liver injury. Curr. Opin. Drug Discov. Devel. 13, 96–103 (2010).
Uetrecht, J. Idiosyncratic drug reactions: current understanding. Annu. Rev. Pharmacol. Toxicol. 47, 513–539 (2007).
Lammert, C. et al. Relationship between daily dose of oral medications and idiosyncratic drug induced liver injury: search for signals. Hepatology 47, 2003–2009 (2008).
Fourches, D. et al. Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species. Chem. Res. Toxicol. 23, 171–183 (2010).
Matthews, E. J. et al. Identification of structure-activity relationships for adverse effects of pharmaceuticals in humans: Part C: use of QSAR and an expert system for the estimation of the mechanism of action of drug-induced hepatobiliary and urinary tract toxicities. Regul. Toxicol. Pharmacol. 54, 43–65 (2009).
Chalasani, N., Teal, E. & Hall, S. D. Effect of rosiglitazone on serum liver biochemistries in diabetic patients with normal and elevated baseline liver enzymes. Am. J. Gastroenterol. 100, 1317–1321 (2005).
Floyd, J. S., Barbehenn, E., Lurie, P. & Wolfe, S. M. Case series of liver failure associated with rosiglitazone and pioglitazone. Pharmacoepidemiol. Drug Saf. 18, 1238–1243 (2009).
Walgren, J. L., Mitchell, M. D. & Thompson, D. C. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 35, 325–361 (2005).
Uetrecht, J. Immunoallergic drug-induced liver injury in humans. Semin. Liver Dis. 29, 383–392 (2009).
Bissell, D. M., Gores, G. J., Laskin, D. L. & Hoofnagle, J. H. Drug-induced liver injury: mechanisms and test systems. Hepatology 33, 1009–1013 (2001).
Bryant, A. E. 3rd & Dreifuss, F. E. Valproic acid hepatic fatalities. III. U.S. experience since 1986. Neurology 46, 465–469 (1996).
McKenzie, R. et al. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 333, 1099–1105 (1995).
Kakuda, T. N. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22, 685–708 (2000).
Stewart, J. D. et al. Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology 52, 1791–1796 (2010).
Fromenty, B. & Pessayre, D. Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol. Ther. 67, 101–154 (1995).
Silva, M. F. et al. Valproate inhibits the mitochondrial pyruvate-driven oxidative phosphorylation in vitro. J. Inherit. Metab. Dis. 20, 397–400 (1997).
Kaas, G. E. & Price, S. C. Role of mitochondria in drug-induced cholestatic injury. Clin. Liver Dis. 12, 27–51 (2008).
Lucena, M. I. et al. Mitochondrial superoxide dismutase and glutathione peroxidase in idiosyncratic drug-induced liver injury. Hepatology 52, 303–312 (2010).
Huang, Y. S. et al. Genetic polymorphisms of manganese superoxide dismutase, NAD(P)H:quinone oxidoreductase, glutathione S-transferase M1 and T1, and the susceptibility to drug-induced liver injury. J. Hepatol. 47, 128–134 (2007).
Hamanishi, T. et al. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular disease in Japanese type 2 diabetic patents. Diabetes 53, 2455–2460 (2004).
Li, M. K. & Crawford, J. M. The pathology of cholestasis. Semin. Liver Dis. 24, 21–42 (2004).
Valayudham, L. S. & Farrell, G. C. Drug-induced cholestasis. Expert Opin. Drug Saf. 2, 287–304 (2003).
Stapelbroek, J. M., van Erpecum, K. J., Klomp, L. W. & Houwen, R. H. Liver disease associated with canalicular transport defects: current and future therapies. J. Hepatol. 52, 258–271 (2010).
Pauli-Magnus, C. & Meier, P. J. Hepatobiliary transporters and drug-induced cholestasis. Hepatology 44, 778–787 (2006).
Watkins, P. B. & Seeff, L. B. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology 43, 618–631 (2006).
Lang, C. et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet. Genomics 17, 47–60 (2007).
Nies, A. T. et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology 50, 1227–1240 (2009).
Degott, C. et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology 15, 244–251 (1992).
Benz, C. et al. Effect of tauroursodeoxycholic acid on bile acid-induced apoptosis in primary human hepatocytes. Eur. J. Clin. Invest. 30, 203–209 (2000).
Andrade, R. J. et al. Drug induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 129, 512–521 (2005).
Bonkovsky, H. L. et al. Immunoallergic manifestations of drug- induced liver injury in the USA. Results from the prospective study of the DILI network [abstract]. Gastroenterology 136 (Suppl. 1), A-820 (2009).
Robin, M. A., Le Roy, M., Descatoire, V. & Pessayre, D. Plasma membrane cytochromes P450 as neoantigens and autoimmune targets in drug-induced hepatitis. J. Hepatol. 26 (Suppl. 1), 23–30 (1997).
Manns, M. P. & Obermayer-Straub, P. Cytochromes P450 and uridine triphosphate-glucuronosyltransferases: model autoantigens to study drug-induced, virus-induced, and autoimmune liver disease. Hepatology 26, 1054–1066 (1997).
Ganey, P. E., Luyendyk, J. P., Maddox, J. F. & Roth, R. A. Adverse hepatic drug reactions: inflammamatory episodes as consequence and contributor. Chem. Biol. Interact. 150, 35–51 (2004).
Gordin, F. M., Simon, G. L., Wofsy, C. B. & Mills, J. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann. Intern. Med. 100, 495–499 (1984).
Watkins, P. B. Biomarkers for the diagnosis and management of drug-induced liver injury. Semin. Liver Dis. 29, 393–399 (2009).
Hautekeete, M. L. et al. HLA association of amoxicillin–clavulanate-induced hepatitis. Gastroenterology 117, 1181–1186 (1999).
O'Donohue, J. et al. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut 47, 717–720 (2000).
Andrade, R. J. et al. HLA class II genotype influences the types of liver injury in drug-induced idiosyncratic liver disease. Hepatology 39, 1603–1612 (2004).
Daly, A. K. et al. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat. Genet. 41, 816–819 (2009).
Mallal, S. et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 358, 568–579 (2008).
Andrews, E. et al. A role for the pregnane X receptor in flucloxacillin-induced liver injury. Hepatology 51, 1656–1664 (2010).
Kindmark, A. et al. Genome-wide pharmacogenetic investigation of a hepatic adverse event without clinical signs of immunopathology suggests and underlying immune pathogenesis. Pharmacogenomics J. 8, 186–195 (2008).
Singer, J. B. et al. A genome-wide study identifies HLA alleles associated with lumiracoxib-related liver injury. Nat. Genet. 42, 711–714 (2010).
Sharp, J. R., Ishak, K. G. & Zimmerman, H. J. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann. Intern. Med. 92, 14–19 (1980).
Gough, A., Chapman, S., Wagstaff, K., Emery, P. & Elias, E. Minocycline-induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. BMJ 312, 169–172 (1996).
Björnsson, E. et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. 51, 2040–2048 (2010).
Papay, J. I. et al. Drug-induced liver injury following positive drug rechallenge. Regul. Toxicol. Pharmacol. 54, 84–90 (2009).
Tahaoglu, K. et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 5, 65–69 (2001).
Hunt, C. M. Mitochondrial and immunoallergic injury increase risk of positive drug rechallenge after drug-induced liver injury: a systematic review. Hepatology 52, 2216–2222 (2010).
Pichler, W. J. & Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 59, 809–820 (2004).
Merk, H. F. Diagnosis of drug hypersensitivity: lymphocyte transformation test and cytokines. Toxicology 209, 217–220 (2005).
Author information
Authors and Affiliations
Contributions
S. Tujios and R. F. Fontana contributed equally to researching data for the article, discussing the content, writing and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. J. Fontana has been a Consultant for GlaxoSmithKline and Vertex Pharmaceuticals, and has been on the Speaker's bureau for Genentech and Gilead Sciences. S. Tujios declares no competing interests.
Rights and permissions
About this article
Cite this article
Tujios, S., Fontana, R. Mechanisms of drug-induced liver injury: from bedside to bench. Nat Rev Gastroenterol Hepatol 8, 202–211 (2011). https://doi.org/10.1038/nrgastro.2011.22
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2011.22
This article is cited by
-
Spatially resolved in vivo imaging of inflammation-associated mRNA via enzymatic fluorescence amplification in a molecular beacon
Nature Biomedical Engineering (2022)
-
Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury
Cell Biology and Toxicology (2022)
-
Pre-clinical 2D and 3D toxicity response to a panel of nanomaterials; comparative assessment of NBM-induced liver toxicity
Drug Delivery and Translational Research (2022)
-
The hepatotoxicity of Polygonum multiflorum: The emerging role of the immune-mediated liver injury
Acta Pharmacologica Sinica (2021)
-
Wdr1 and cofilin are necessary mediators of immune-cell-specific apoptosis triggered by Tecfidera
Nature Communications (2021)