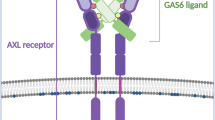
Abstract
The clearance of apoptotic cells is critical for both tissue homeostasis and the resolution of inflammation. We found that the TAM receptor tyrosine kinases Axl and Mer had distinct roles as phagocytic receptors in these two settings, in which they exhibited divergent expression, regulation and activity. Mer acted as a tolerogenic receptor in resting macrophages and during immunosuppression. In contrast, Axl was an inflammatory response receptor whose expression was induced by proinflammatory stimuli. Axl and Mer differed in their ligand specificities, ligand-receptor complex formation in tissues, and receptor shedding upon activation. These differences notwithstanding, phagocytosis by either protein was strictly dependent on receptor activation triggered by bridging of TAM receptor–ligand complexes to the 'eat-me' signal phosphatidylserine on the surface of apoptotic cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Muñoz, L.E., Lauber, K., Schiller, M., Manfredi, A.A. & Herrmann, M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 6, 280–289 (2010).
Lemke, G. Biology of the TAM receptors. Cold Spring Harbor Perspectives 5, a009076 (2013).
Lemke, G. & Rothlin, C.V. Immunobiology of the TAM receptors. Nat. Rev. Immunol. 8, 327–336 (2008).
Lu, Q. & Lemke, G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science 293, 306–311 (2001).
Rothlin, C.V., Ghosh, S., Zuniga, E.I., Oldstone, M.B. & Lemke, G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136 (2007).
Burstyn-Cohen, T. et al. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron 76, 1123–1132 (2012).
Scott, R.S. et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 (2001).
Lemke, G. & Burstyn-Cohen, T. TAM receptors and the clearance of apoptotic cells. Ann. NY Acad. Sci. 1209, 23–29 (2010).
Lu, Q. et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature 398, 723–728 (1999).
Bhattacharyya, S. et al. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe 14, 136–147 (2013).
Meertens, L. et al. TIM and TAM receptors mediate dengue virus infection. Cell Host Microbe 12, 544–557 (2012).
Paolino, M. et al. Essential role of E3 ubiquitin ligase activity in Cbl-b-regulated T cell functions. J. Immunol. 186, 2138–2147 (2011).
Schlegel, J. et al. MERTK receptor tyrosine kinase is a therapeutic target in melanoma. J. Clin. Invest. 123, 2257–2267 (2013).
Meyer, A.S., Miller, M.A., Gertler, F.B. & Lauffenburger, D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 6, ra66 (2013).
Carrera Silva, E.A. et al. T cell-derived protein S engages TAM receptor signaling in dendritic cells to control the magnitude of the immune response. Immunity 39, 160–170 (2013).
Inaba, K. et al. Isolation of dendritic cells. Curr. Protoc. Immunol. 25, 3.7 (2009).
Rahman, Z.S., Shao, W.H., Khan, T.N., Zhen, Y. & Cohen, P.L. Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses. J. Immunol. 185, 5859–5868 (2010).
Subramanian, M. et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J. Clin. Invest. 124, 1296–308 (2014).
McColl, A. et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. J. Immunol. 183, 2167–2175 (2009).
A-Gonzalez, N. et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009).
Mukundan, L. et al. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat. Med. 15, 1266–1272 (2009).
Clark, A.R. Anti-inflammatory functions of glucocorticoid-induced genes. Mol. Cell. Endocrinol. 275, 79–97 (2007).
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Feng, X. et al. Lipopolysaccharide inhibits macrophage phagocytosis of apoptotic neutrophils by regulating the production of tumour necrosis factor alpha and growth arrest-specific gene 6. Immunology 132, 287–295 (2011).
Nishi, C., Toda, S., Segawa, K. & Nagata, S. Tim4- and MerTK- mediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol. Cell. Biol. 34, 1512–1520 (2014).
Seitz, H.M., Camenisch, T.D., Lemke, G., Earp, H.S. & Matsushima, G.K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 178, 5635–5642 (2007).
Miksa, M., Komura, H., Wu, R., Shah, K.G. & Wang, P. A novel method to determine the engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. J. Immunol. Methods 342, 71–77 (2009).
Oka, K. et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl. Acad. Sci. USA 95, 9535–9540 (1998).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007).
Schroeder, G.M. et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J. Med. Chem. 52, 1251–1254 (2009).
Lemmon, M.A. & Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 (2010).
Kahn, C.R., Baird, K.L., Jarrett, D.B. & Flier, J.S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc. Natl. Acad. Sci. USA 75, 4209–4213 (1978).
Schreiber, A.B., Libermann, T.A., Lax, I., Yarden, Y. & Schlessinger, J. Biological role of epidermal growth factor-receptor clustering. Investigation with monoclonal anti-receptor antibodies. J. Biol. Chem. 258, 846–853 (1983).
Todt, J.C., Hu, B. & Curtis, J.L. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J. Leukoc. Biol. 75, 705–713 (2004).
O'Bryan, J.P., Fridell, Y.W., Koski, R., Varnum, B. & Liu, E.T. The transforming receptor tyrosine kinase, Axl, is post-translationally regulated by proteolytic cleavage. J. Biol. Chem. 270, 551–557 (1995).
Costa, M., Bellosta, P. & Basilico, C. Cleavage and release of a soluble form of the receptor tyrosine kinase ARK in vitro and in vivo. J. Cell. Physiol. 168, 737–744 (1996).
Ekman, C., Site, D.F., Gottsater, A., Lindblad, B. & Dahlback, B. Plasma concentrations of growth arrest specific protein 6 and the soluble form of its tyrosine kinase receptor Axl as markers of large abdominal aortic aneurysms. Clin. Biochem. 43, 110–114 (2010).
Zhu, H. et al. Different expression patterns and clinical significance of mAxl and sAxl in systemic lupus erythematosus. Lupus 23, 624–634 (2014).
Ko, C.P., Yu, Y.L., Hsiao, P.C., Yang, S.F. & Yeh, C.B. Plasma levels of soluble Axl correlate with severity of community-acquired pneumonia. Mol. Med. Rep. 9, 1400–1404 (2014).
Liu, X. et al. Plasma concentrations of sAxl are associated with severe preeclampsia. Clin. Biochem. 47, 173–176 (2014).
Lee, C.H. et al. Plasma concentrations predict aortic expression of growth-arrest-specific protein 6 in patients undergoing coronary artery bypass grafting. PLoS ONE 8, e79452 (2013).
Hsiao, F.C. et al. Circulating growth arrest-specific 6 protein is associated with adiposity, systemic inflammation, and insulin resistance among overweight and obese adolescents. J. Clin. Endocrinol. Metab. 98, E267–E274 (2013).
Camenisch, T.D., Koller, B.H., Earp, H.S. & Matsushima, G.K. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J. Immunol. 162, 3498–3503 (1999).
Sen, P. et al. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-κB activation in dendritic cells. Blood 109, 653–660 (2007).
Casanova-Acebes, M. et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035 (2013).
Scheiermann, C., Kunisaki, Y. & Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 13, 190–198 (2013).
Holland, S.J. et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 70, 1544–1554 (2010).
Ye, X. et al. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene 29, 5254–5264 (2010).
Rothlin, C.V. & Lemke, G. TAM receptor signaling and autoimmune disease. Curr. Opin. Immunol. 22, 740–746 (2010).
van den Brand, B.T. et al. Therapeutic efficacy of Tyro3, Axl, and Mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis Rheum. 65, 671–680 (2013).
Angelillo-Scherrer, A. et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat. Med. 7, 215–221 (2001).
Zhang, X., Goncalves, R. & Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 83, 14.1 (2008).
Fourgeaud, L. et al. The metabotropic glutamate receptor mGluR5 is endocytosed by a clathrin-independent pathway. J. Biol. Chem. 278, 12222–12230 (2003).
Acknowledgements
We thank M. Joens and J. Fitzpatrick for processing samples and acquiring scanning electron microscopic images; R. Evans (Salk Institute) for nuclear receptor agonists T0901317, GW501516 and BRL49653; J. Hash, P. Burrola and C. Mayer for technical support; and members of the Lemke laboratory and the Nomis Center for discussions. Supported by the US National Institutes of Health (R01 AI077058, R01 AI101400 and R01 NS085296 to G.L.), the Leona M. and Harry B. Helmsley Charitable Trust (2012-PG-MED002 to G.L.) the Nomis Foundation, the H.N. and Frances C. Berger Foundation, the Fritz B. Burns Foundation, the HKT Foundation, Françoise Gilot-Salk, the Human Frontiers Science Program (A.Z.), the Marie Curie Seventh Framework Programme (P.G.T.), the Leukemia and Lymphoma Society and the Nomis Foundation (E.D.L.).
Author information
Authors and Affiliations
Contributions
A.Z. designed and performed the experiments; P.G.T. aided in the design and execution of in vivo experiments; E.D.L. prepared purified recombinant GAS-6; I.D. aided in the design of the flow-cytometry-based phagocytosis assay; G.L. contributed to the design of the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.L. is a shareholder in Kolltan Pharmaceuticals.
Integrated supplementary information
Supplementary Figure 1 Expression of Axl and Mer on populations of cells of the immune system in vivo.
Immunohistochemistry of Axl and Mer in spleen (a), liver (b), and lung (c). Closed arrowheads: cells co-expressing Axl and Mer; open arrowheads: cells expressing mostly Axl. In the spleen, the principal Axl-Mer co-expressing cells are F4/80+ red pulp (RP) macrophages. In the splenic white pulp (WP) (d), Tingible body macrophages (F4/80-CD68+) express Mer and low levels of Axl (closed arrowheads), whereas a subpopulation of splenic CD11c+ DCs express only Axl (open arrowheads). In the lung (c and e), alveolar macrophages (CD11c+CD11b-MHCII-F4/80lo) are only Axl-positive (open arrowheads). In the liver, F4/80+ Kupffer cells are both Axl and Mer positive (b, closed arrowheads). Bars, 50 μm (a-c); 10 μm (d); and 20 μm (e). Representative images from n=3 mice.
Supplementary Figure 2 Discrete Axl+ and Mer+ cell populations in vitro.
(a) Unstimulated (Ctrl) or IFN-γ (250 U/ml, 18 h) treated BMDM cultures were stained live with Mer (green) and Axl (magenta) antibodies. Closed arrows: cells expressing mostly Axl; open arrows- cells expressing mostly Mer. The asterisk marks a single cell that is weakly positive for both Axl and Mer. Bar, 20 μm. Representative images of three independent experiments.
(b,c) BMDM cultures were stimulated with 0.1 μM Dex or 10 μg/ml poly(I:C) for 24 h, fixed and stained with Mer (b) or Axl (c) antibodies and counterstained with Phalloidin-TRITC. Bar, 100 μm. Representative images of three independent experiments.
Supplementary Figure 3 Regulation of Axl and Mer by steroid hormones.
BMDM cultures were stimulated with 1 μM Dex, hydrocortisone, cortisone, aldosterone, 17β-estradiol, estrone, estriol or progesterone for 24 h. Axl and Mer expression was assayed by immunoblotting. Representative of two independent experiments.
Supplementary Figure 4 Anti-inflammatory effects of Dex are independent of Mer and Axl.
(a) RT-PCR showing the kinetics of Mertk, Fpr1, Mrc1 mRNA induction and Axl and Il21r mRNA inhibition in BMDMs in response to 0.1 μM Dex. (b) BMDMs from indicated mice were treated with 100 ng/ml LPS with or without 0.1 μM Dex. TNF secretion was measured 24 h later by ELISA in culture supernatants. Representative of three independent experiments. (c) RT-PCR showing changes in Tnf, Mfge8, Il21r and Mrc1 mRNAs in BMDMs after 24 h incubation with 0.1 μM Dex. Data in (a) and (c) are normalized to Hprt mRNA and presented as fold change relative to untreated cells. Average of two independent experiments, each done in technical duplicate, graphed as mean ± s.d. (d) Immunoblot showing changes in activity of Akt, ERK1/2 and p38 signaling pathways in response to 0.1 μM Dex treatment of BMDMs derived from indicated knock-out mice. Representative of two independent experiments.
Supplementary Figure 5 Induction of Axl by poly(I:C) and IFN-α in BMDMs.
Cells were incubated with either poly(I:C) (1 μg/ml) or IFN-α (250 U/ml) for the indicated times in hours (h), and then blotted for total Axl (top), Mer (middle), or GAPDH (bottom). Representative of two independent experiments.
Supplementary Figure 6 Flow cytometry–based phagocytosis assay.
Apoptotic cells are labeled with pH-sensitive dye, pHrodo. Once engulfed into the acidic environment of phagosomes, pHrodo fluorescence is enhanced and phagocytic macrophages are distinguished based on their side scatter (SSC-A) and pHrodo fluorescence intensity using flow cytometry. In this experiment, the percent cells in the phagocytic gate is quantified in an 1-hour assay, in absence or presence of 10 nM GAS-6. Representative plot of 6 independent experiments.
Supplementary Figure 7 Regulation of the expression of Axl and Mer and phagocytosis in BMDCs.
(a) BMDC cultures from the indicated mice were stimulated for 10 min with 10 nM GAS-6 (G) or 25 nM Protein S (S). Receptor activation was assayed by immunoprecipitation and immunoblotting. Representative of two independent experiments. (b) BMDCs were cultured for 18 h in the presence of 0.1 μM Dex or 10 μg/ml poly(I:C) and then stimulated for 10 min with 10 nM GAS-6. Receptor activation was assayed by immunoprecipitation and immunoblotting. Representative of two independent experiments. (c, d) BMDCs from mice of the indicated genotypes were cultured for 24 h in the presence of 0.1 μM Dex (c) or 10 μg/ml poly(I:C) (d) and then incubated for 1 h with pHrodo stained ACs with or without 10 nM GAS-6. Percent of phagocytosis was measured using flow cytometry. Data are presented as mean ± s.d. from two independent experiments, each done for duplicate cultures for each condition.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 11409 kb)
Rights and permissions
About this article
Cite this article
Zagórska, A., Través, P., Lew, E. et al. Diversification of TAM receptor tyrosine kinase function. Nat Immunol 15, 920–928 (2014). https://doi.org/10.1038/ni.2986
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2986
This article is cited by
-
Ozone alleviates MSU-induced acute gout pain via upregulating AMPK/GAS6/MerTK/SOCS3 signaling pathway
Journal of Translational Medicine (2023)
-
Receptor tyrosine kinases Tyro3, Axl, and Mertk differentially contribute to antibody-induced arthritis
Cell Communication and Signaling (2023)
-
Axl alleviates DSS-induced colitis by preventing dysbiosis of gut microbiota
Scientific Reports (2023)
-
Regulation of brain endothelial cell physiology by the TAM receptor tyrosine kinase Mer
Communications Biology (2023)
-
Therapeutic targeting of the functionally elusive TAM receptor family
Nature Reviews Drug Discovery (2023)