Abstract
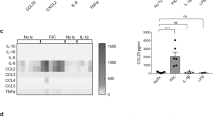
Interleukin 17C (IL-17C) is a member of the IL-17 family that is selectively induced in epithelia by bacterial challenge and inflammatory stimuli. Here we show that IL-17C functioned in a unique autocrine manner, binding to a receptor complex consisting of the receptors IL-17RA and IL-17RE, which was preferentially expressed on tissue epithelial cells. IL-17C stimulated epithelial inflammatory responses, including the expression of proinflammatory cytokines, chemokines and antimicrobial peptides, which were similar to those induced by IL-17A and IL-17F. However, IL-17C was produced by distinct cellular sources, such as epithelial cells, in contrast to IL-17A, which was produced mainly by leukocytes, especially those of the TH17 subset of helper T cells. Whereas IL-17C promoted inflammation in an imiquimod-induced skin-inflammation model, it exerted protective functions in dextran sodium sulfate–induced colitis. Thus, IL-17C is an essential autocrine cytokine that regulates innate epithelial immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Abraham, C. & Medzhitov, R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 140, 1729–1737 (2011).
Nestle, F.O., Di Meglio, P., Qin, J.Z. & Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Saenz, S.A., Taylor, B.C. & Artis, D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol. Rev. 226, 172–190 (2008).
Sims, J.E. & Smith, D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Kishimoto, T. IL-6: from its discovery to clinical applications. Int. Immunol. 22, 347–352 (2010).
Jager, A. & Kuchroo, V.K. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand. J. Immunol. 72, 173–184 (2010).
Blaschitz, C. & Raffatellu, M. Th17 cytokines and the gut mucosal barrier. J. Clin. Immunol. 30, 196–203 (2010).
Kolls, J.K. & Khader, S.A. The role of Th17 cytokines in primary mucosal immunity. Cytokine Growth Factor Rev. 21, 443–448 (2010).
Ahmed, M. & Gaffen, S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 21, 449–453 (2010).
Iwakura, Y., Ishigame, H., Saijo, S. & Nakae, S. Functional specialization of interleukin-17 family members. Immunity 34, 149–162 (2011).
Hymowitz, S.G. et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341 (2001).
Ely, L.K., Fischer, S. & Garcia, K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 10, 1245–1251 (2009).
Liang, S.C. et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799 (2007).
Wright, J. F. et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282, 13447–13455 (2007).
Fort, M.M. et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15, 985–995 (2001).
Lee, J. et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 276, 1660–1664 (2001).
Rickel, E.A. et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 181, 4299–4310 (2008).
Shi, Y. et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J. Biol. Chem. 275, 19167–19176 (2000).
Liu, Y. et al. IL-17A and TNF-α exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. J. Immunol. 186, 3197–3205 (2011).
Aujla, S.J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 (2008).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Khader, S.A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Ogawa, A., Andoh, A., Araki, Y., Bamba, T. & Fujiyama, Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62 (2004).
Yang, X. O. et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205, 1063–1075 (2008).
Fujino, S. et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52, 65–70 (2003).
Johansen, C. et al. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 160, 319–324 (2009).
Leipe, J. et al. Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum. 62, 2876–2885 (2010).
Lock, C. et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 8, 500–508 (2002).
Hofstetter, H. H. et al. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 237, 123–130 (2005).
Hueber, W. et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72 (2010).
Lubberts, E. et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50, 650–659 (2004).
Rizzo, H.L. et al. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J. Immunol. 186, 1495–1502 (2011).
Holland, D.B., Bojar, R.A., Farrar, M.D. & Holland, K.T. Differential innate immune responses of a living skin equivalent model colonized by Staphylococcus epidermidis or Staphylococcus aureus. FEMS Microbiol. Lett. 290, 149–155 (2009).
Johansen, C., Riis, J.L., Gedebjerg, A., Kragballe, K. & Iversen, L. Tumor necrosis factor α-mediated induction of interleukin 17C in human keratinocytes is controlled by nuclear factor kB. J. Biol. Chem. 286, 25487–25494 (2011).
Wu, Q. et al. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 9, 78–86 (2007).
Hurst, S.D. et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169, 443–453 (2002).
Li, H. et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. USA 97, 773–778 (2000).
Yamaguchi, Y. et al. IL-17B and IL-17C are associated with TNF-α production and contribute to the exacerbation of inflammatory arthritis. J. Immunol. 179, 7128–7136 (2007).
Spriggs, M.K. Interleukin-17 and its receptor. J. Clin. Immunol. 17, 366–369 (1997).
Li, T.S., Li, X.N., Chang, Z.J., Fu, X.Y. & Liu, L. Identification and functional characterization of a novel interleukin 17 receptor: a possible mitogenic activation through ras/mitogen-activated protein kinase signaling pathway. Cell. Signal. 18, 1287–1298 (2006).
Chiricozzi, A. et al. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Invest. Dermatol. 131, 677–687 (2011).
Kao, C.Y. et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-kB signaling pathways. J. Immunol. 173, 3482–3491 (2004).
Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 10, 131–144 (2010).
Apostolaki, M., Armaka, M., Victoratos, P. & Kollias, G. Cellular mechanisms of TNF function in models of inflammation and autoimmunity. Curr. Dir. Autoimmun. 11, 1–26 (2010).
Van Maele, L. et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3negCD127+ immune cells in spleen and mucosa. J. Immunol. 185, 1177–1185 (2010).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Ma, H.L. et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 118, 597–607 (2008).
van der Fits, L. et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 182, 5836–5845 (2009).
Reiter, M.J., Testerman, T.L., Miller, R.L., Weeks, C.E. & Tomai, M.A. Cytokine induction in mice by the immunomodulator imiquimod. J. Leukoc. Biol. 55, 234–240 (1994).
Chiang, E.Y. et al. Targeted depletion of lymphotoxin-α-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat. Med. 15, 766–773 (2009).
Acknowledgements
We thank R. Neupane and A. Paler-Martinez for cell sorting; A. Abbas for microarray assistance; and J. Ngo and R. Asuncion for mouse colony maintenance.
Author information
Authors and Affiliations
Contributions
V.R.-C. did most of the experiments and analyzed the data; A.S. contributed to identifying the receptors and sources of IL-17C; E.L. and L.G. did the radioligand-binding experiments; Z.L. and M.B. contributed to the imiquimod-induced skin inflammation studies; S.J., J.Lesch, L.D. and J.d.V. did the DSS studies; J.H. analyzed the microarray data; J.K. and M.X. contributed to the adenoviral studies; M.Z. and W.L. did the intradermal IL-17C injections; J.Lai and T.S. generated the mouse antibody to mouse IL-17C; Z.M. contributed to the microarray studies; P.C. contributed to the histological evaluation of the adenoviral, intradermal injection and imiquimod studies; H.S. and W.O. provided scientific input for the project; and R.P. devised and planned the project and wrote the manuscript
Corresponding author
Ethics declarations
Competing interests
All authors are employees of Genentech.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Table 1 and Methods (PDF 8478 kb)
Rights and permissions
About this article
Cite this article
Ramirez-Carrozzi, V., Sambandam, A., Luis, E. et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol 12, 1159–1166 (2011). https://doi.org/10.1038/ni.2156
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2156
This article is cited by
-
Cytokine profile in first-episode drug-naïve major depressive disorder patients with or without anxiety
BMC Psychiatry (2024)
-
IL-17C is a driver of damaging inflammation during Neisseria gonorrhoeae infection of human Fallopian tube
Nature Communications (2024)
-
Management of Moderate to Severe Plaque Psoriasis with Brodalumab in Daily Practice: Real-World Evidence from the LIBERO Study in the Czech Republic
Dermatology and Therapy (2024)
-
Circulatory Inflammatory Proteins as Early Diagnostic Biomarkers for Invasive Aspergillosis in Patients with Hematologic Malignancies—an Exploratory Study
Mycopathologia (2024)
-
The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment
Cellular & Molecular Immunology (2023)