Abstract
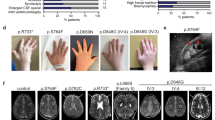
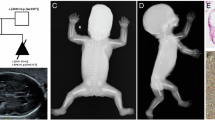
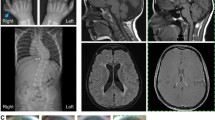
Protein-protein interaction analyses have uncovered a ciliary and basal body protein network that, when disrupted, can result in nephronophthisis (NPHP), Leber congenital amaurosis, Senior-Løken syndrome (SLSN) or Joubert syndrome (JBTS)1,2,3,4,5,6. However, details of the molecular mechanisms underlying these disorders remain poorly understood. RPGRIP1-like protein (RPGRIP1L) is a homolog of RPGRIP1 (RPGR-interacting protein 1), a ciliary protein defective in Leber congenital amaurosis7,8. We show that RPGRIP1L interacts with nephrocystin-4 and that mutations in the gene encoding nephrocystin-4 (NPHP4) that are known to cause SLSN disrupt this interaction. RPGRIP1L is ubiquitously expressed, and its protein product localizes to basal bodies. Therefore, we analyzed RPGRIP1L as a candidate gene for JBTS and identified loss-of-function mutations in three families with typical JBTS, including the characteristic mid-hindbrain malformation. This work identifies RPGRIP1L as a gene responsible for JBTS and establishes a central role for cilia and basal bodies in the pathophysiology of this disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Mollet, G. et al. The gene mutated in juvenile nephronophthisis type 4 encodes a novel protein that interacts with nephrocystin. Nat. Genet. 32, 300–305 (2002).
Otto, E.A. et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat. Genet. 37, 282–288 (2005).
Roepman, R. et al. Interaction of nephrocystin-4 and RPGRIP1 is disrupted by nephronophthisis or Leber congenital amaurosis-associated mutations. Proc. Natl. Acad. Sci. USA 102, 18520–18525 (2005).
Sayer, J.A. et al. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat. Genet. 38, 674–681 (2006).
Parisi, M.A. et al. The NPHP1 gene deletion associated with juvenile nephronophthisis is present in a subset of individuals with Joubert syndrome. Am. J. Hum. Genet. 75, 82–91 (2004).
den Hollander, A.I. et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. Am. J. Hum. Genet. 79, 556–561 (2006).
Roepman, R. et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum. Mol. Genet. 9, 2095–2105 (2000).
Dryja, T.P. et al. Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am. J. Hum. Genet. 68, 1295–1298 (2001).
Satran, D., Pierpont, M.E. & Dobyns, W.B. Cerebello-oculo-renal syndromes including Arima, Senior-Loken and COACH syndromes: more than just variants of Joubert syndrome. Am. J. Med. Genet. 86, 459–469 (1999).
Parisi, M.A., Doherty, D., Chance, P.F. & Glass, I.A. Joubert syndrome (and related disorders) (OMIM 213300). Eur. J. Hum. Genet. 15, 511–521 (2007).
Maria, B.L. et al. Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J. Child Neurol. 14, 368–376 (1999).
Pellegrino, J.E., Lensch, M.W., Muenke, M. & Chance, P.F. Clinical and molecular analysis in Joubert syndrome. Am. J. Med. Genet. 72, 59–62 (1997).
Ferland, R.J. et al. Abnormal cerebellar development and axonal decussation due to mutations in AHI1 in Joubert syndrome. Nat. Genet. 36, 1008–1013 (2004).
Baala, L. et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. Am. J. Hum. Genet. 80, 186–194 (2007).
Fliegauf, M. et al. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. J. Am. Soc. Nephrol. 17, 2424–2433 (2006).
Valente, E.M. et al. Mutations in CEP290, which encodes a centrosomal protein, cause pleiotropic forms of Joubert syndrome. Nat. Genet. 38, 623–625 (2006).
Dawe, H.R. et al. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum. Mol. Genet. 16, 173–186 (2007).
Chang, B. et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum. Mol. Genet. 15, 1847–1857 (2006).
Otto, E. et al. A gene mutated in nephronophthisis and retinitis pigmentosa encodes a novel protein, nephroretinin, conserved in evolution. Am. J. Hum. Genet. 71, 1161–1167 (2002).
Wolfrum, U. Centrin in the photoreceptor cells of mammalian retinae. Cell Motil. Cytoskeleton 32, 55–64 (1995).
Rattner, A., Chen, J. & Nathans, J. Proteolytic shedding of the extracellular domain of photoreceptor cadherin. Implications for outer segment assembly. J. Biol. Chem. 279, 42202–42210 (2004).
Banizs, B. et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development 132, 5329–5339 (2005).
Doolin, P.F. & Birge, W.J. Ultrastructural organization of cilia and basal bodies of the epithelium of the choroid plexus in the chick embryo. J. Cell Biol. 29, 333–345 (1966).
Ramensky, V., Bork, P. & Sunyaev, S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 30, 3894–3900 (2002).
Ng, P.C. & Henikoff, S. Predicting deleterious amino acid substitutions. Genome Res. 11, 863–874 (2001).
Kantardzhieva, A. et al. MPP5 recruits MPP4 to the CRB1 complex in photoreceptors. Invest. Ophthalmol. Vis. Sci. 46, 2192–2201 (2005).
Roepman, R., Schick, D. & Ferreira, P.A. Isolation of retinal proteins that interact with retinitis pigmentosa GTPase regulator by interaction trap screen in yeast. Methods Enzymol. 316, 688–704 (2000).
van Wijk, E. et al. The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum. Mol. Genet. 15, 751–765 (2006).
Brandstatter, J.H., Koulen, P., Kuhn, R., van der, P.H. & Wassle, H. Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. J. Neurosci. 16, 4749–4756 (1996).
Mogensen, M.M., Malik, A., Piel, M., Bouckson-Castaing, V. & Bornens, M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J. Cell Sci. 113, 3013–3023 (2000).
Acknowledgements
We thank A.I. den Hollander and H. Kremer for discussions, the University of Washington Center for Ecogenetics and Environmental Health (CEEH) for SNP mapping, C. Hutter for biostatistical advice and C. Beumer, S. van der Velde-Visser, G. Merkx and E. Sehn for expert technical assistance. We thank all the participating families with Joubert syndrome and S. Aysun, P.F. Chance, D. Knutzen and J. Adkins for clinical and technical support. We thank Y. Xiumin (Department of Cell Biology, Max Planck Institute of Biochemistry) for the antibodies to CEP290 and ninein; J. Salisbury (Department of Biochemistry and Molecular Biology, Mayo Clinic College of Medicine) for the anti-pan-centrin and B.K. Yoder (Department of Cell Biology, University of Alabama at Birmingham Medical Center) for rabbit anti-polaris. This work was supported by grants from the Dutch Kidney Foundation (C04.2112 to N.K. and R.R.), Pro Retina Germany (to R.R.), EVI-GENORET (LSHG-CT-2005 512036 to F.P.M.C. and R.R.), Rotterdamse Vereniging Blindenbelangen (to F.P.M.C. and R.R.), Stichting Blindenhulp (to F.P.M.C. and R.R.), Stichting OOG (to F.P.M.C. and R.R.), Algemene Nederlandse Vereniging ter Voorkoming van Blindheid (to F.P.M.C. and R.R.), Landelijke Stichting voor Blinden en Slechtzienden (to A.K. and F.P.M.C.), FAUN-Stiftung (to U.W.), Forschung contra Blindheit (to T.M. and U.W.), the US National Institutes of Health (grants K23-NS45832 to M.A.P., K24-HD46712 to I.A.G., and K12-RR023265 to D.D., the University of Washington Center on Human Development and Disability (NICHD P30 HD02274 to D.D., M.A.P. and I.A.G.), the University of Washington CEEH, the US National Institute of Environmental Health Sciences (P30ES07033 to F.M.F.) and the March of Dimes Endowment for Healthier Babies at Children's Hospital in Seattle to D.D., M.A.P. and I.A.G.).
Author information
Authors and Affiliations
Contributions
The study was designed by N.V.A.M.K. and R.R., who share senior authorship of this paper. H.H.A., S.E.C.v.B., S.J.F.L., T.A.P., T.M., A.K. and U.W. analyzed protein expression and localization; T.M. and U.W. performed immuno-electron microscopy; H.H.A. and S.J.F.L. analyzed protein-protein interactions; S.E.C.v.B. analyzed gene expression; M.A.P., H.O., H.Y.K. and N.V.A.M.K. acquired clinical data; D.D., N.T.G and F.M.F. performed mapping of microsatellites and SNPs; S.E.C.v.B., N.T.G. and K.V. performed sequence analysis of families; D.D., H.G.B., F.P.M.C., I.A.G., N.V.A.M.K. and R.R. supervised the work and H.H.A., D.D. and R.R. wrote the manuscript, with assistance from most of the coauthors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Reciprocal GST pulldown analysis of RPGRIP1L and nephrocystin-4. (PDF 319 kb)
Supplementary Fig. 2
Localization of eCFP-nephrocystin-4 and mRFP-RPGRIP1LC2-N-end in COS-1 cells. (PDF 531 kb)
Supplementary Fig. 3
Confirmation of the specificity of the anti-RPGRIP1L antibody SNC040. (PDF 364 kb)
Supplementary Fig. 4
Confirmation of the specificity of staining by the anti-RPGRIP1L antibody SNC040 in the retina, brain and kidney. (PDF 121 kb)
Supplementary Fig. 5
Localization of RPGRIP1L and nephrocystin-4 in the retina. (PDF 167 kb)
Supplementary Fig. 6
Joubert syndrome–associated mutations in RPGRIP1L disrupt the interaction with nephrocystin-4. (PDF 209 kb)
Supplementary Table 1
Affected individuals with RPGRIP1L mutations show features of Joubert syndrome and Meckel-Gruber syndrome. (PDF 95 kb)
Supplementary Table 2
Oligonucleotide primers used for RT-PCR and sequencing of RPGRIP1L and for RT-PCR of RPGRIP1, NPHP4 and GAPDH. (PDF 87 kb)
Rights and permissions
About this article
Cite this article
Arts, H., Doherty, D., van Beersum, S. et al. Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 39, 882–888 (2007). https://doi.org/10.1038/ng2069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng2069